Issue Archive
Table of Contents
EDITORIAL
Introduction to a review series on mantle cell lymphoma: sands shifting in the darkness
Mantle cell lymphoma (MCL) is a rare and aggressive form of non-Hodgkin B-cell lymphoma that defies cures in most patients. Over the last decade, significant advances have been made in our understanding of the biology of the disease, patient risk stratification, and novel therapies. Associate Editor Philippe Armand presents a series of reviews that provide advice on management of patients, illuminate current understanding of the molecular landscape associated with the disease, and how that may shape future research and care. Ryan et al review the current frontline management of MCL in a rapidly evolving field, underscoring ongoing phase 3 trials; Silkenstedt and Dreyling highlight the emerging treatments for relapsed and refractory MCL; Jain and Wang tackle the critical issue of risk assignment based on clinical and molecular features; and Sarkozy and colleagues explore in depth the biology of MCL with an emphasis not only on intrinsic vulnerabilities but also on the tumor microenvironment.
BLOOD COMMENTARIES
REVIEW SERIES
Frontline management of mantle cell lymphoma
Mantle cell lymphoma (MCL) is a rare and aggressive form of non-Hodgkin B-cell lymphoma that defies cures in most patients. Over the last decade, significant advances have been made in our understanding of the biology of the disease, patient risk stratification, and novel therapies. Associate Editor Philippe Armand presents a series of reviews that provide advice on management of patients, illuminate current understanding of the molecular landscape associated with the disease, and how that may shape future research and care. Ryan et al review the current frontline management of MCL in a rapidly evolving field, underscoring ongoing phase 3 trials; Silkenstedt and Dreyling highlight the emerging treatments for relapsed and refractory MCL; Jain and Wang tackle the critical issue of risk assignment based on clinical and molecular features; and Sarkozy and colleagues explore in depth the biology of MCL with an emphasis not only on intrinsic vulnerabilities but also on the tumor microenvironment.
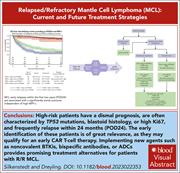
Treatment of relapsed/refractory MCL
Clinical Trials & Observations
Mantle cell lymphoma (MCL) is a rare and aggressive form of non-Hodgkin B-cell lymphoma that defies cures in most patients. Over the last decade, significant advances have been made in our understanding of the biology of the disease, patient risk stratification, and novel therapies. Associate Editor Philippe Armand presents a series of reviews that provide advice on management of patients, illuminate current understanding of the molecular landscape associated with the disease, and how that may shape future research and care. Ryan et al review the current frontline management of MCL in a rapidly evolving field, underscoring ongoing phase 3 trials; Silkenstedt and Dreyling highlight the emerging treatments for relapsed and refractory MCL; Jain and Wang tackle the critical issue of risk assignment based on clinical and molecular features; and Sarkozy and colleagues explore in depth the biology of MCL with an emphasis not only on intrinsic vulnerabilities but also on the tumor microenvironment.
High-risk MCL: recognition and treatment
Mantle cell lymphoma (MCL) is a rare and aggressive form of non-Hodgkin B-cell lymphoma that defies cures in most patients. Over the last decade, significant advances have been made in our understanding of the biology of the disease, patient risk stratification, and novel therapies. Associate Editor Philippe Armand presents a series of reviews that provide advice on management of patients, illuminate current understanding of the molecular landscape associated with the disease, and how that may shape future research and care. Ryan et al review the current frontline management of MCL in a rapidly evolving field, underscoring ongoing phase 3 trials; Silkenstedt and Dreyling highlight the emerging treatments for relapsed and refractory MCL; Jain and Wang tackle the critical issue of risk assignment based on clinical and molecular features; and Sarkozy and colleagues explore in depth the biology of MCL with an emphasis not only on intrinsic vulnerabilities but also on the tumor microenvironment.
Unraveling MCL biology to understand resistance and identify vulnerabilities
Mantle cell lymphoma (MCL) is a rare and aggressive form of non-Hodgkin B-cell lymphoma that defies cures in most patients. Over the last decade, significant advances have been made in our understanding of the biology of the disease, patient risk stratification, and novel therapies. Associate Editor Philippe Armand presents a series of reviews that provide advice on management of patients, illuminate current understanding of the molecular landscape associated with the disease, and how that may shape future research and care. Ryan et al review the current frontline management of MCL in a rapidly evolving field, underscoring ongoing phase 3 trials; Silkenstedt and Dreyling highlight the emerging treatments for relapsed and refractory MCL; Jain and Wang tackle the critical issue of risk assignment based on clinical and molecular features; and Sarkozy and colleagues explore in depth the biology of MCL with an emphasis not only on intrinsic vulnerabilities but also on the tumor microenvironment.
CLINICAL TRIALS AND OBSERVATIONS
Long-term 3-year follow-up of mosunetuzumab in relapsed or refractory follicular lymphoma after ≥2 prior therapies
Clinical Trials & Observations
In relapsed/refractory follicular lymphoma (R/R FL), achieving remission is not sufficient, as the main challenge is maintaining long-term response without significant toxicity. Sehn and colleagues report the long-term follow-up (3 years) of fixed duration therapy with mosunetuzumab, a bispecific antibody that engages T cells and lymphoma cells, in a phase 1/2 study in patients with R/R FL, finding that the majority of complete responses are maintained beyond 2 years and that normal B-cell recovery occurs after a median of 18 months. With few late toxicities observed, these data support the use of mosunetuzumab as a safe and effective treatment for R/R FL.
IMMUNOBIOLOGY AND IMMUNOTHERAPY
CD70 CAR T cells secreting an anti-CD33/anti-CD3 dual-targeting antibody overcome antigen heterogeneity in AML
Chimeric antigen receptor (CAR) T-cell therapy for acute myeloid leukemia (AML) is confounded by antigen heterogeneity and antigen escape. Silva et al report the development of a novel dual-targeting CAR-T that engages CD70 and secretes a bispecific antibody that not only targets CD33 but also recruits bystander T cells to attack CD33+ AML cells. Using in vitro and in vivo models, the authors show increased cytotoxicity, improved tumor control, and increased persistence of these cells, justifying further research to assess efficacy in patients and potential immune-related toxicities and other off-target effects.
LYMPHOID NEOPLASIA
Heme promotes venetoclax resistance in multiple myeloma through MEK-ERK signaling and purine biosynthesis
The B-cell lymphoma 2 (BCL-2) inhibitor venetoclax is highly active against multiple myeloma (MM) with the t(11;14) translocation; however, some patients develop drug resistance, leading to relapse. Nair et al report that heme plays a critical role in the sensitivity of MM cells to venetoclax, showing that increased heme promotes resistance while inhibition of heme biosynthesis increases sensitivity and that this is driven by activation of MEK-ERK signaling and increased purine biosynthesis. The study uncovers a novel metabolic vulnerability in MM cells that can be potentially targeted, amplifying the effects of BCL2 inhibition.
MYELOID NEOPLASIA
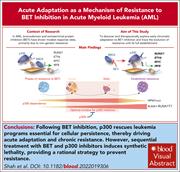
Acute resistance to BET inhibitors remodels compensatory transcriptional programs via p300 coactivation
Bromodomain and extraterminal (BET) bromodomain-containing protein 4 (BRD4) is a therapeutic target in acute myeloid leukemia (AML), but inhibitors show only modest clinical success due to toxicity and resistance when used as monotherapy. Analyzing the epigenomic effect of BRD4/BET inhibition, Shah et al identified a compensatory prosurvival transcriptional feedback loop led by the lysine acetyltransferase coactivator protein p300 that enables tolerance to BET inhibition by suppressing expression of genes critical for AML maintenance. Utilizing both cellular and animal models, the investigators demonstrated that dual inhibition of BET and p300 disrupts this compensatory feedback, specifically when sequential dosing is employed, and suggest this combinatorial approach for future clinical trials.
RED CELLS, IRON, AND ERYTHROPOIESIS
Ferroptosis regulates hemolysis in stored murine and human red blood cells
During storage in blood banks, red blood cells (RBCs) accumulate oxidative damage that make them more susceptible to hemolysis, leading to poor transfusion outcomes. With a comprehensive genetic and metabolic approach, using well-phenotyped mice and human cohorts, D’Alessandro and colleagues provide new insights into the mechanism of RBC “storage lesions.” The authors identify a ferroptosis-like process modulated by Steap3 as a mechanism of red cell damage and provide a potential platform for pharmacological intervention.
BLOOD WORK
-
Cover Image
Cover Image
![issue cover]()
Photomicrograph of bone marrow from an immunocompromised mouse engrafted with human Molm13 acute myeloid leukemia, then treated with CD70-targeted chimeric antigen receptor (CAR) T cells that secrete a CD33 T-cell engaging antibody. Immunohistochemistry for human CD3 (brown) demonstrates expansion of the CAR T cells in the bone marrow. See the article by Silva et al on page 720.
- PDF Icon Front MatterFront Matter
- PDF Icon Table of ContentsTable of Contents
- PDF Icon Editorial BoardEditorial Board
Advertisement intended for health care professionals
Email alerts
Advertisement intended for health care professionals










Mosunetuzumab next up to bat … is it a home run?
Clinical Trials & Observations