Despite immunosuppressive prophylaxis, about a third of patients undergoing allogeneic hematopoietic cell transplantation (alloHCT) develop chronic graft-versus-host disease (cGVHD), a leading cause of long-term morbidity in transplant survivors. Most established risk factors for cGVHD (eg, donor-recipient HLA mismatch, use of peripheral blood as the stem cell source, female-to-male transplant, older patient age) are not modifiable after transplantation. Modifiable clinical or laboratory features predictive of cGVHD risk based on each patient’s posttransplant experience could inform novel strategies to prevent cGVHD. In this issue of Blood, Habenicht et al have identified one such feature, the gut microbiota.1
Disruptions to commensal microbial communities (also known as dysbiosis) are common and often profound in alloHCT recipients.2 Early posttransplant dysbiosis has been repeatedly associated with acute GVHD risk.3,4 Fewer studies have evaluated the possibility of a similar connection in cGVHD.5-8 Habenicht et al examined whether B-cell dysregulation, a previously established hallmark of cGVHD,9 might start long before cGVHD onset, thereby increasing the likelihood of a causal contribution to cGVHD pathogenesis. They first demonstrated clonal expansion of plasmablasts at day +90 in patients subsequently developing cGVHD, as compared with those without GVHD or with aGVHD only. In multivariable analysis adjusting for clinically established predictors of cGVHD, plasmablast frequency at day +90 remained a significant correlate of cGVHD. Next, the authors showed that the expanded plasmablasts (analyzed at day +180) predominantly produced immunoglobulin A (IgA) isotypes with molecular evidence for recent generation in mucosal sites and capable of binding to members of the commensal gut microbiota. The main bacterial genera recognized by the expanded IgA clones were Faecalibacterium, Blautia, Bacteroides, and Ruminococcus, which are abundant members of a healthy gut microbiota.
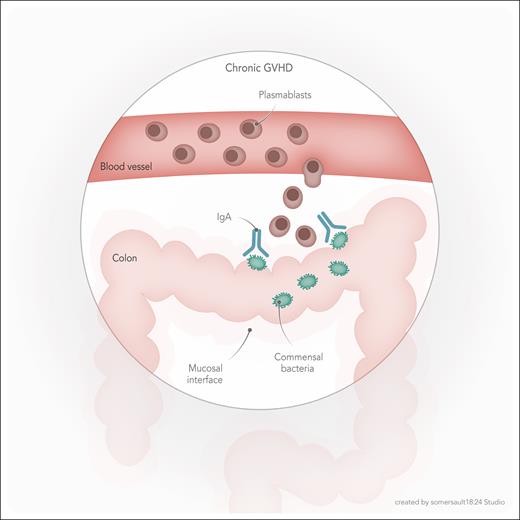
Overall, data presented by Habenicht et al suggest a model in which bacterial antigenic stimulation at the intestinal mucosal barrier, likely potentiated by local inflammation, results in an IgA antibody response, including clonal expansion, hypermutation, and selection of plasmablasts, followed by their entry to the blood via the lymphatic system, and eventually, contribution to cGVHD pathogenesis (see figure). Findings from this elegant study advance the field by demonstrating evidence for a novel mechanism connecting gut dysbiosis with cGVHD. An earlier mechanistic report finding a role for early microbiota changes in cGVHD pathogenesis focused on the oral microbiota.5 Oral dysbiosis in a murine model resulted in expansion of the Enterococcaceae family and its migration to cervical lymph nodes, activation of antigen-presenting cells, and expansion of donor-derived inflammatory T cells. The study by Habenicht et al is the first, to my knowledge, to show a plasmablast-mediated connection between the microbiota and cGVHD.
Model for gut microbiota mediation of chronic GVHD. Early posttransplant IgA antibody response to commensal bacteria in the colon leads to clonal expansion of plasmablasts, associated with a higher risk for future cGVHD. The precise mechanism by which the process of bacterial recognition (and possibly immune-mediated clearance), IgA production, and clonal plasmablast expansion may lead to cGVHD is unknown.
Model for gut microbiota mediation of chronic GVHD. Early posttransplant IgA antibody response to commensal bacteria in the colon leads to clonal expansion of plasmablasts, associated with a higher risk for future cGVHD. The precise mechanism by which the process of bacterial recognition (and possibly immune-mediated clearance), IgA production, and clonal plasmablast expansion may lead to cGVHD is unknown.
A brand-new niche has now been opened for microbiota research to make a clinical impact in transplant survivors. However, several questions remain to be answered. First, can expanded circulating plasmablasts reactive to gut commensal bacteria be used as a biomarker for early identification of high-risk patients for cGVHD? Findings by Habenicht et al suggest so. However, external validation is needed to ascertain this possibility. Second, whether and how immunosuppressive medications used for GVHD prophylaxis and treatment might influence the frequency of plasmablasts is unknown. This important variable needs to be studied and adjusted for in future analyses. Third, microbiota profiling using short-amplicon sequencing has limited resolution beyond genus. Thus, the species or strain specificity of the observed microbial reactivity of plasmablasts is unknown, limiting the ability to precisely determine the mechanisms involved. Fourth, and perhaps most importantly, although the temporal sequence of events observed in this work, that is microbiota changes then plasmablast expansion and then cGVHD, suggests causality, it does not prove it. Assuming that causality will be demonstrated in future animal studies with fewer clinical covariates than in the complex setting of patients with alloHCT, at least 2 scenarios can be envisioned. The commensal bacteria recognized by plasmablast-derived IgA may have protective effects against cGVHD, for example, via producing immunomodulatory short-chain fatty acids such as butyrate. Clearance of these bacteria following immune recognition could lead to loss of protective metabolites and subsequently cGVHD. In the second scenario, the expanded plasmablasts or their microbial-specific IgA clones could directly influence target tissues and facilitate the development of cGVHD.
The potential value of day +90 plasmablast frequency as a biomarker for cGVHD risk should be tested in other studies. If this relationship is demonstrated in independent cohorts, high-risk patients may be candidates for adjusted prophylactic interventions such as prolonged or delayed immunosuppression withdrawal, or at least closer clinical follow-up. Research over the last decade has demonstrated causal links between microbiota and acute GVHD, inspiring recent microbiota therapeutic trials attempting to prevent acute GVHD without escalating immunosuppressive medications.10 The study by Habenicht et al introduces a novel provocative concept, taking us toward cGVHD as another transplant complication potentially amenable to microbiota-targeted interventions. This would require a more precise characterization of the microbial species involved and their direct or indirect effect on cGVHD target tissues.
Conflict-of-interest disclosure: A.R. has received consulting fees from Seres Therapeutics (2023) and is a paid member of an Emmes data and safety monitoring board.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal