Issue Archive
Table of Contents
Targeting STK17B kinase activates ferroptosis and suppresses drug resistance in multiple myeloma
The progression of multiple myeloma (MM) is often associated with the suppression of ferroptosis, a type of cell death driven by iron-dependent lipid peroxidation. Yan et al investigated mechanisms underlying MM resistance to ferroptosis and found that STK17B phosphorylates key regulators of iron homeostasis, thereby maintaining a balance between proferroptotic and antiferroptotic proteins. Pharmacological inhibition of STK17B activates ferroptosis, overcomes MM drug resistance, and reduces tumor burden in mouse models, suggesting a potential targeted therapy to overcome drug resistance in myeloma.
Tissue transglutaminase drives fibrin β-chain cross-linking: a novel fibrin modification observed in patients with trauma
Clinical Trials & Observations
Brief Report
Tissue transglutaminase (TG2) may modify fibrin(ogen) cross-linking during tissue injury, but its contribution to pathological clot formation in trauma remains unclear. Boateng et al identified novel β-chain cross-links involving Q82 in fibrin clots from trauma patient plasma that are absent in normal controls. In vitro, TG2 modifies fibrin(ogen) cross-linking and reproduces the same β-α cross-links observed in clots from patients with trauma. Defining these TG2-specific cross-links provides insight into previously unrecognized fibrin modifications in the setting of trauma.
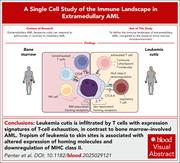
Mechanisms of immune escape and extramedullary tropism in leukemia cutis
There are limited data on the mechanisms underlying skin infiltration in extramedullary acute myeloid leukemia (AML). Penter et al used bulk and single-cell transcriptomic profiling to delineate pathways associated with skin tropism and the unique microenvironment of leukemia cutis. They identified T cells exhibiting exhaustion signatures that are distinct from those seen in bone marrow–involved AML, as well as altered expression of homing molecules and downregulation of major histocompatibility class II. Their analysis suggests that extramedullary AML arises from a complex interplay between leukemia-intrinsic features and local immune effector cell dysfunction, suggesting potential therapeutic strategies.
Fetal context conveys heritable protection against MLL-rearranged AML that depends on MLL3
Although MLL rearrangements drive most infant leukemias and can occur before birth with few cooperating mutations, the incidence of congenital leukemia is remarkably low. Mendoza-Castrejon et al used sophisticated mouse models and multiomic profiling to show a heritable, fetal-stage resistance to leukemic transformation that is enforced by the epigenetic regulator MLL3. Identification of MLL3 as a key enforcer of this resistance adds a critical layer to our understanding of pediatric acute myeloid leukemia (AML) and offers new insights into the developmental origins of leukemia.
Genomic determinants of response and resistance to pirtobrutinib in relapsed/refractory chronic lymphocytic leukemia
Clinical Trials & Observations
Targeting Bruton tyrosine kinase (BTK) has transformed chronic lymphocytic leukemia (CLL) therapy, but resistance remains a major problem. Pirtobrutinib, a noncovalent, reversible BTK inhibitor (BTKi), is active in patients with CLL resistant to covalent BTKis. Brown et al analyzed CLL cells from patients with relapsed/refractory CLL in the phase 1/2 BRUIN trial and defined mutations that emerged during pirtobrutinib treatment. Of note, one-third of patients showed no BTK mutations at progression, underscoring the complexity of resistance.
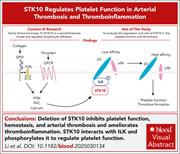
STK10 regulates platelet function in arterial thrombosis and thromboinflammation
Serine/threonine kinase 10 (STK10) is a serine/threonine kinase and regulates lymphocyte adhesion. Li et al evaluated the expression and role of STK10 in platelet function and thrombosis, demonstrating that deletion of STK10 inhibits platelet function, hemostasis, and arterial thrombosis and ameliorates thromboinflammation. Mechanistically, STK10 interacts with integrin-linked protein kinase and phosphorylates it to regulate platelet function. This newly defined role for STK10 in platelet functions offers potential therapeutic opportunities.
Measurable residual disease intervention in AML: a new therapeutic horizon
Clinical Trials & Observations
Although measurable residual disease (MRD) is an established prognostic tool in acute myeloid leukemia (AML), its clinical utility is limited by the absence of a universal MRD marker. In this Perspective, Wei et al report the conceptual and statistical underpinnings of INTERCEPT, a multitarget, multiarm platform trial designed to adaptively expand the range of both MRD markers and MRD-directed therapeutic strategies. This provides a foundation for prospectively integrating MRD into therapeutic decision-making and potentially transforming management of AML.
EDITORIAL
BLOOD COMMENTARIES
ERRATUM
-
Cover Image
Cover Image
![issue cover]()
Cover art by Brian Cannon, American Society of Hematology.
- PDF Icon Front MatterFront Matter
- PDF Icon Table of ContentsTable of Contents
- PDF Icon Editorial BoardEditorial Board
Advertisement intended for health care professionals
Email alerts
Advertisement intended for health care professionals







Targeting STK17B: unlocking ferroptosis in myeloma