Key Points
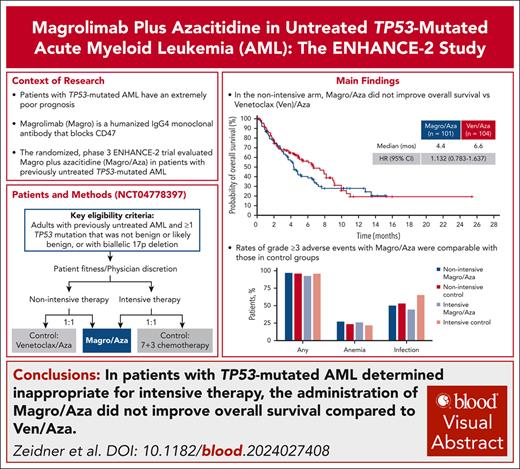
In the ENHANCE-2 study, Magro/Aza did not improve OS of patients with TP53-mutated AML.
Rates of grade ≥3 adverse events with Magro/Aza were comparable with those in the control groups.
Visual Abstract
Patients with TP53-mutated acute myeloid leukemia (AML) have an extremely poor prognosis, necessitating new treatments. The global, randomized, phase 3 ENHANCE-2 trial evaluated the anti-CD47 monoclonal antibody magrolimab plus azacitidine (Magro/Aza) for previously untreated TP53-mutated AML. Patients determined ineligible for intensive therapy were randomized to receive Magro/Aza or venetoclax plus Aza (Ven/Aza); those eligible for intensive therapy were randomized to receive Magro/Aza or 7+3 induction chemotherapy. The primary end point was overall survival (OS) in the nonintensive arm. At interim analysis, nonintensive-arm OS hazard ratio (HR) between treatment groups was 1.191 (95% confidence interval [CI], 0.744-1.906), meeting the study’s definition for futility and resulting in study termination. At final analysis, median OS was 4.4 vs 6.6 months (HR, 1.132; 95% CI, 0.783-1.637; P = .5070) in the nonintensive arm (n = 205) and 7.3 vs 11.1 months (HR, 1.434; 95% CI, 0.635-3.239; P = .3798) in the intensive arm (n = 52) between Magro/Aza and control groups, respectively. Incidences of grade ≥3 adverse events were similar across Magro/Aza and control groups (nonintensive, n = 194: 96.9% and 95.9%; intensive, n = 50: 92.6% and 95.7%), including grade ≥3 anemia (nonintensive: 27.1% and 23.5%; intensive: 25.9% and 21.7%). Grade ≥3 infections were observed in 50.0% and 53.1% of patients in the nonintensive arm and 44.4% and 65.2% of intensive-arm patients. ENHANCE-2 did not meet its primary end point of OS in TP53-mutated AML but provides important data informing future studies in this challenging population. This trial was registered at www.clinicaltrials.gov as #NCT04778397.
Introduction
Acute myeloid leukemia (AML) is a clonal myeloid malignancy affecting predominantly older adults, with a 5-year estimated survival of 31.9%.1 Intensive chemotherapy (IC) remains a standard treatment for young and fit patients with newly diagnosed AML2; however, most newly diagnosed patients are aged >65 years1 and often ineligible for IC.2 Venetoclax (Ven; a B-cell lymphoma 2 antagonist) combined with hypomethylating agents (most commonly azacitidine [Aza]) or low-dose cytarabine has become a standard of care for patients with AML who are ineligible for IC.2-4
TP53 mutations occur in ∼7% to 18% of patients with AML (30%-50% of therapy-related cases)5-7 and are associated with therapy resistance.7 Regardless of frontline therapy (intensive or nonintensive), outcomes are dismal for patients with TP53-mutated AML (median overall survival [OS], 6.1-6.5 months).8,9 This continued poor prognosis despite new AML treatments is exemplified by the phase 3 VIALE-A study, which compared Ven plus Aza (Ven/Aza) with placebo plus Aza (placebo/Aza) for patients with newly diagnosed AML who were ineligible for standard induction therapy.2 A pooled subset analysis including patients with TP53 mutation from VIALE-A and an early-stage study (ClinicalTrials.gov identifier: NCT02203773) revealed no significant difference in clinical outcomes between Ven/Aza and placebo/Aza (median OS, 5.5 vs 5.4 months).10 Poor outcomes and distinct biology associated with TP53 mutation have led to International Consensus Classification recognition of TP53-mutated AML as a distinct clinical entity, highlighting the need for novel, better tailored treatment options.11
Magrolimab (Magro) is a humanized immunoglobulin G4 monoclonal antibody that blocks CD47.12 Magro is postulated to promote immunogenic cancer cell death.13,14 Aza increases CD47 and calreticulin (phagocytosis activator) expression, providing mechanistic rationale for combining Magro with Aza (Magro/Aza).12 In vitro coculture experiments of a TP53-mutant AML cell line with human macrophages showed that Magro/Aza treatment increased AML cell phagocytosis compared with Aza monotherapy.12 In a phase 1b (ClinicalTrials.gov identifier: NCT03248479) study in previously untreated AML, Magro/Aza was well tolerated and demonstrated promising efficacy (objective response rate [ORR], 47.2%; complete remission [CR] rate, 31.9%; median OS, 9.8 months) in TP53-mutated AML (n = 72).15 Of the 11.5% who proceeded to stem cell transplant (SCT), median OS was not reached.15 The ENHANCE-2 study evaluated the efficacy and safety of Magro/Aza vs physician’s choice of Ven/Aza (nonintensive therapy) or 7+3 chemotherapy (intensive therapy) for patients with previously untreated TP53-mutated AML.
Methods
Patient population
This phase 3, randomized, open-label, multicenter study (ClinicalTrials.gov identifier: NCT04778397) enrolled patients with previously untreated, histologically confirmed AML (by World Health Organization classification) and ≥1 TP53 mutation that was not benign or not likely benign, or with biallelic 17p deletion. Patients were tested for TP53 mutations in bone marrow (BM) aspirate cells using central or local next-generation sequencing per institutional standards. Results from patients enrolled based on local testing were reviewed and confirmed by central review before randomization.
Eligible patients were aged ≥18 years; had an Eastern Cooperative Oncology Group performance status (ECOG PS) score of 0 to 2 (patients aged <75 years and eligible for nonintensive therapy could have an ECOG PS of 0-3); and had adequate kidney, liver, and cardiac function. A hemoglobin level of ≥9 g/dL was required before the first 2 doses of any study treatment and the first 2 doses of Magro. Red blood cell transfusions were allowed to meet hemoglobin eligibility. Postinfusion hemoglobin monitoring was required 3 to 6 hours after initiation of the first and second doses of Magro during initial treatment. Patients with acute promyelocytic leukemia or clinical suspicion of active central nervous system involvement with leukemia were excluded. Full inclusion and exclusion criteria are provided in the supplemental Methods and Protocol, available on the Blood website.
Randomization and treatment
Before randomization, it was determined by investigators whether patients were eligible for nonintensive or intensive therapy. Reasons for choosing nonintensive therapy were age, ECOG PS, comorbidity, or investigator discretion. Patients eligible for nonintensive therapy were randomized 1:1 to receive Magro/Aza or Ven/Aza (control group). Patients eligible for intensive therapy were randomized 1:1 to receive Magro/Aza or 7+3 induction chemotherapy (control group). Randomization was stratified by eligibility for nonintensive vs intensive therapy, age (age of <75 vs ≥75 years), and geographic region (United States vs non–United States; supplemental Figure 1).
Treatment was administered in 28-day cycles. Magro was administered IV at a dose of 1 mg/kg on days 1 and 4 (priming); 15 mg/kg on day 8; and 30 mg/kg on days 11 and 15, followed by 5 weekly 30-mg/kg doses, and then administered at 30 mg/kg every 2 weeks starting 1 week after the last weekly dose. Ven was given orally at 100 mg on day 1, followed by 200 mg on day 2 and 400 mg daily thereafter. Aza 75 mg/m2 was administered subcutaneously or IV on days 1 through 7 (or as 7 doses over 9 consecutive days) of each 28-day cycle.
For the intensive-arm control group, daunorubicin (60 mg/m2 IV) or idarubicin (12 mg/m2 IV) was given on days 1 through 3 along with continuous infusion of cytarabine (100 or 200 mg/m2 IV) on days 1 through 7. For patients requiring reinduction after BM assessment of response at cycle 1, day 15, administration of daunorubicin (60 mg/m2 IV) or idarubicin (12 mg/m2 IV) on days 1 through 2 along with continuous infusion of cytarabine (100 or 200 mg/m2 IV) on days 1 through 5 was allowed (“5+2” regimen). Consolidation therapy consisted of cytarabine 1500 or 3000 mg/m2 IV (1000 mg/m2 IV was permitted for patients aged ≥60 years based on local practice) every 12 hours on days 1, 3, and 5, for up to 4 cycles.
Reasons considered sufficient for treatment discontinuation included but were not limited to SCT, unacceptable toxicity, progressive disease (PD), loss of clinical benefit, or death. Dose modifications and delays were allowed per protocol. Magro and Aza dosing could be decoupled if Aza was delayed because of adverse events (AEs) not considered related to Magro to allow Magro to continue per the protocol-defined dosing schedule.
Procedures
Nonintensive-arm BM response assessments were conducted at screening and on day 28 of cycles 1, 2, 4, and 6, and then every 3 cycles thereafter. Intensive-arm control group BM response assessments were done at screening; cycle 1, day 15; and at count recovery after induction therapy or day 42 after the start of the most recent induction treatment, whichever was earlier. During consolidation, BM response was obtained at count recovery for the cycle. When consolidation therapy was stopped, a response assessment was performed within 42 days after day 1 of the most recent consolidation cycle. For patients undergoing SCT, response assessments were continued every 12 weeks from the date of SCT until documented PD, relapse, or initiation of new therapy. Measurable residual disease was assessed centrally in BM aspirates using multiparameter flow cytometry for AML with a positivity threshold of <0.1%. Next-generation sequencing and red blood cell phenotyping procedures are provided in the supplemental Methods.
Outcomes
The primary end point was OS in patients eligible for nonintensive therapy. Secondary end points included OS in all patients; event-free survival (EFS; time from date of randomization to the earliest date of relapse, treatment failure, or death from any cause) in all patients; rates of CR, CR without measurable residual disease, and CR with partial hematologic recovery (CRh) within 6 months for nonintensive therapy or 2 months for 7+3 chemotherapy; duration of CR; duration of CR + CRh; incidence of grade ≥3 treatment-emergent AEs (TEAEs) and laboratory abnormalities; Magro serum concentration; and anti-Magro antibody incidence rate. Treatment failure was defined as failure to achieve CR within either 6 months (nonintensive therapy) or 2 months (7+3 chemotherapy) of treatment. Exploratory end points are listed in the supplemental Methods and Protocol.
Hematologic responses of CR, morphologic CR with incomplete blood count recovery (CRi), and partial remission (PR) were assessed following European LeukemiaNet 2017 recommendations16 and based on investigator assessment. Stable disease was defined as not meeting criteria for any other treatment response (ie, CR, CRi, PR, and PD). Hematologic improvement was assessed according to International Working Group 2006 criteria.17 ORR was the proportion of patients who reached CR + CRi + CRh + PR + morphologic leukemia-free state. Response rates within 6 months of treatment (2 months for 7+3 chemotherapy) were determined. TEAEs were defined as new or worsening AEs between the time of first dose and the end of the safety follow-up period (70 days after last dose of study treatment) or initiation of new anti-AML therapy, whichever was first, and were graded according to Common Terminology Criteria for AEs version 5.0.
Trial oversight
All patients provided written informed consent before study participation. The study was conducted according to International Conference on Harmonisation good clinical practice guidelines, the Declaration of Helsinki, and local institutional review board requirements. An external multidisciplinary data monitoring committee was established to review the study’s progress and perform interim reviews of safety data regularly.
Statistical analysis
The intent-to-treat (ITT) analysis set included all randomized patients and was the primary analysis set for efficacy. The safety population included all patients who received ≥1 dose of study drug. Interim futility and superiority analyses of OS were planned when ∼69 and 128 deaths had occurred, respectively, in the stratum of patients eligible for nonintensive therapy; this corresponded to 40% and 75%, respectively, of the expected 171 deaths required for the primary analysis. Sample sizes are described in the supplemental Methods.
Interim futility analysis included a stratified log-rank test for OS in patients eligible for nonintensive therapy and was reviewed by the external data monitoring committee. A nonbinding futility rule with boundary hazard ratio (HR) of 1.1 was implemented. Given a true OS HR of 0.74 in patients eligible for nonintensive therapy, the probability of observing a HR of >1.1 at interim analysis was <5%.
A log-rank test stratified by randomization stratification factors was used to compare OS between treatment arms. A stratified Cox proportional hazard regression model was used to estimate HR and its 2-sided 95% confidence interval (CI). The Kaplan-Meier method was used to estimate median OS and its 95% CI.
Results
Patients
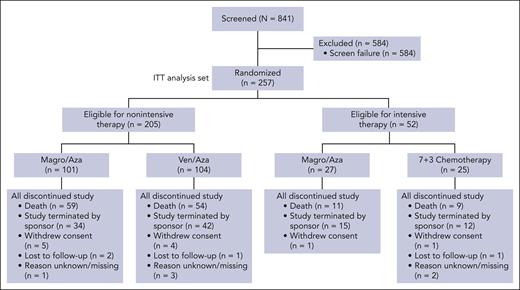
From July 2021 through May 2024, 257 of 346 planned patients were randomized and included in the ITT population (nonintensive arm, n = 205; intensive arm, n = 52; Figure 1). Median patient age in the nonintensive arm was older than that in the intensive arm but well balanced between treatment groups (Table 1). No patients aged ≥75 years were deemed eligible for intensive therapy. In the nonintensive arm, 34.7% and 34.6% of patients were aged ≥75 years in Magro/Aza and Ven/Aza groups, respectively. The most common reasons for nonintensive therapy were age (69.3% vs 65.4%) and a reason other than physical fitness (23.8% vs 20.2%) and ECOG PS (16.8% vs 15.4%; supplemental Table 1). Other baseline characteristics were well balanced across treatment arms (Table 1). The most common reasons for study discontinuation in both nonintensive and intensive arms were death and study termination (Figure 1); the most common cause of death in both arms was PD.
CONSORT diagram of the ENHANCE-2 trial. 7+3 chemotherapy, 7 days of continuous IV cytarabine (100 or 200 mg/m2 daily) and 3 days of IV daunorubicin (60 mg/m2 daily) or idarubicin (12 mg/m2 daily) on days 1 through 3.
CONSORT diagram of the ENHANCE-2 trial. 7+3 chemotherapy, 7 days of continuous IV cytarabine (100 or 200 mg/m2 daily) and 3 days of IV daunorubicin (60 mg/m2 daily) or idarubicin (12 mg/m2 daily) on days 1 through 3.
Patient characteristics in the ENHANCE-2 trial
| Characteristic . | Eligible for nonintensive therapy . | Eligible for intensive therapy . | ||
|---|---|---|---|---|
| Magro/Aza (n = 101) . | Ven/Aza (n = 104) . | Magro/Aza (n = 27) . | 7+3 chemotherapy∗ (n = 25) . | |
| Age, median (range), y | 71 (27-87) | 72 (54-88) | 57 (27-73) | 64 (41-74) |
| Age group, n (%) | ||||
| <75 y | 66 (65.3) | 68 (65.4) | 27 (100) | 25 (100) |
| ≥75 y | 35 (34.7) | 36 (34.6) | 0 | 0 |
| Sex, n (%) | ||||
| Male | 58 (57.4) | 61 (58.7) | 22 (81.5) | 14 (56.0) |
| Female | 43 (42.6) | 43 (41.3) | 5 (18.5) | 11 (44.0) |
| Geographical region, n (%) | ||||
| United States | 31 (30.7) | 33 (31.7) | 1 (3.7) | 0 |
| Outside United States | 70 (69.3) | 71 (68.3) | 26 (96.3) | 25 (100) |
| ECOG PS, n (%) | ||||
| 0 | 26 (25.7) | 33 (31.7) | 14 (51.9) | 13 (52.0) |
| 1 | 50 (49.5) | 43 (41.3) | 9 (33.3) | 9 (36.0) |
| 2 | 19 (18.8) | 20 (19.2) | 3 (11.1) | 0 |
| 3 | 1 (1.0) | 1 (1.0) | 0 | 0 |
| Missing | 5 (5.0) | 7 (6.7) | 1 (3.7) | 3 (12.0) |
| AML subtype, n (%) | ||||
| Myelodysplasia related | 45 (44.6) | 42 (40.4) | 13 (48.1) | 11 (44.0) |
| Therapy related | 19 (18.8) | 26 (25.0) | 5 (18.5) | 3 (12.0) |
| Cytogenetic risk assessment, n (%) | ||||
| Intermediate | 12 (11.9) | 8 (7.7) | 2 (7.4) | 3 (12.0) |
| Adverse | 66 (65.3) | 67 (64.4) | 22 (81.5) | 16 (64.0) |
| Unknown/missing | 21 (20.8) | 28 (26.9) | 3 (11.1) | 6 (24.0) |
| TP53 mutations,†n (%) | ||||
| Monoallelic | 13 (12.9) | 15 (14.4) | 2 (7.4) | 2 (8.0) |
| Biallelic‡ | 77 (76.2) | 74 (71.1) | 22 (81.5) | 20 (80.0) |
| Missing data§ | 11 (10.9) | 15 (14.4) | 3 (11.1) | 3 (12.0) |
| Cytogenetics detected,||n (%) | ||||
| Monosomal | 8 (7.9) | 11 (10.6) | 6 (22.2) | 4 (16.0) |
| Complex karyotype | 14 (13.9) | 22 (21.2) | 5 (18.5) | 3 (12.0) |
| −17/17p | 8 (7.9) | 8 (7.7) | 2 (7.4) | 1 (4.0) |
| Other | 62 (61.4) | 56 (53.8) | 16 (59.3) | 12 (48.0) |
| Unknown/missing | 12 (11.9) | 14 (13.5) | 1 (3.7) | 4 (16.0) |
| BM blast, n (%) | ||||
| <30% | 57 (56.4) | 63 (60.6) | 18 (66.7) | 13 (52.0) |
| ≥30% to <50% | 23 (22.8) | 15 (14.4) | 3 (11.1) | 6 (24.0) |
| ≥50% | 16 (15.8) | 18 (17.3) | 5 (18.5) | 4 (16.0) |
| Missing | 5 (5.0) | 8 (7.7) | 1 (3.7) | 2 (8.0) |
| Characteristic . | Eligible for nonintensive therapy . | Eligible for intensive therapy . | ||
|---|---|---|---|---|
| Magro/Aza (n = 101) . | Ven/Aza (n = 104) . | Magro/Aza (n = 27) . | 7+3 chemotherapy∗ (n = 25) . | |
| Age, median (range), y | 71 (27-87) | 72 (54-88) | 57 (27-73) | 64 (41-74) |
| Age group, n (%) | ||||
| <75 y | 66 (65.3) | 68 (65.4) | 27 (100) | 25 (100) |
| ≥75 y | 35 (34.7) | 36 (34.6) | 0 | 0 |
| Sex, n (%) | ||||
| Male | 58 (57.4) | 61 (58.7) | 22 (81.5) | 14 (56.0) |
| Female | 43 (42.6) | 43 (41.3) | 5 (18.5) | 11 (44.0) |
| Geographical region, n (%) | ||||
| United States | 31 (30.7) | 33 (31.7) | 1 (3.7) | 0 |
| Outside United States | 70 (69.3) | 71 (68.3) | 26 (96.3) | 25 (100) |
| ECOG PS, n (%) | ||||
| 0 | 26 (25.7) | 33 (31.7) | 14 (51.9) | 13 (52.0) |
| 1 | 50 (49.5) | 43 (41.3) | 9 (33.3) | 9 (36.0) |
| 2 | 19 (18.8) | 20 (19.2) | 3 (11.1) | 0 |
| 3 | 1 (1.0) | 1 (1.0) | 0 | 0 |
| Missing | 5 (5.0) | 7 (6.7) | 1 (3.7) | 3 (12.0) |
| AML subtype, n (%) | ||||
| Myelodysplasia related | 45 (44.6) | 42 (40.4) | 13 (48.1) | 11 (44.0) |
| Therapy related | 19 (18.8) | 26 (25.0) | 5 (18.5) | 3 (12.0) |
| Cytogenetic risk assessment, n (%) | ||||
| Intermediate | 12 (11.9) | 8 (7.7) | 2 (7.4) | 3 (12.0) |
| Adverse | 66 (65.3) | 67 (64.4) | 22 (81.5) | 16 (64.0) |
| Unknown/missing | 21 (20.8) | 28 (26.9) | 3 (11.1) | 6 (24.0) |
| TP53 mutations,†n (%) | ||||
| Monoallelic | 13 (12.9) | 15 (14.4) | 2 (7.4) | 2 (8.0) |
| Biallelic‡ | 77 (76.2) | 74 (71.1) | 22 (81.5) | 20 (80.0) |
| Missing data§ | 11 (10.9) | 15 (14.4) | 3 (11.1) | 3 (12.0) |
| Cytogenetics detected,||n (%) | ||||
| Monosomal | 8 (7.9) | 11 (10.6) | 6 (22.2) | 4 (16.0) |
| Complex karyotype | 14 (13.9) | 22 (21.2) | 5 (18.5) | 3 (12.0) |
| −17/17p | 8 (7.9) | 8 (7.7) | 2 (7.4) | 1 (4.0) |
| Other | 62 (61.4) | 56 (53.8) | 16 (59.3) | 12 (48.0) |
| Unknown/missing | 12 (11.9) | 14 (13.5) | 1 (3.7) | 4 (16.0) |
| BM blast, n (%) | ||||
| <30% | 57 (56.4) | 63 (60.6) | 18 (66.7) | 13 (52.0) |
| ≥30% to <50% | 23 (22.8) | 15 (14.4) | 3 (11.1) | 6 (24.0) |
| ≥50% | 16 (15.8) | 18 (17.3) | 5 (18.5) | 4 (16.0) |
| Missing | 5 (5.0) | 8 (7.7) | 1 (3.7) | 2 (8.0) |
NGS, next-generation sequencing.
Seven days of continuous IV cytarabine (100 or 200 mg/m2 daily) and 3 days of IV daunorubicin (60 mg/m2 daily) or idarubicin (12 mg/m2 daily) on days 1 through 3.
Based on targeted NGS.
One patient had both NGS and biallelic 17p deletion results.
Refers to missing NGS data; these patients were enrolled based on 17p deletion testing.
Cytogenetics were locally collected and not centrally reviewed.
All enrolled patients had TP53-mutated AML, with most having biallelic TP53 alterations and few with an identified monoallelic TP53 alteration status. Between Magro/Aza and Ven/Aza in the nonintensive arm, monoallelic TP53 alterations were identified in 12.9% and 14.4%, respectively, whereas biallelic alterations were identified in 76.2% and 71.1%, respectively.
Efficacy
Nonintensive arm
An interim futility analysis was conducted with a clinical cutoff date of 11 August 2023, when 73 of a planned 171 deaths had occurred in the nonintensive arm (43% event fraction). The OS HR for Magro/Aza vs Ven/Aza was 1.191 (95% CI, 0.744-1.906); median OS was 4.4 vs 7.4 months; and median duration of OS follow-up was 2 vs 2.12 months. Based on these results, the study was deemed futile and terminated. After discontinuation of all patients from study treatment, a final analysis was performed. At final analysis (data cutoff date: 14 May 2024), 118 deaths had occurred in the nonintensive arm. Median duration of follow-up for OS in was 3.65 vs 4.07 months with Magro/Aza vs Ven/Aza, and median OS was 4.4 vs 6.6 months (HR, 1.132; 95% CI, 0.783-1.637; Figure 2). Three of 101 patients (3.0%) in the Magro/Aza group and 8 of 104 patients (7.7%) in the Ven/Aza group received SCT. Ad hoc analysis of OS based on monoallelic vs biallelic TP53 mutation revealed similar results as in the entire nonintensive-arm population (supplemental Figure 2).
EFS results are shown in supplemental Figure 3. ORRs were 23.8% vs 51.0% in Magro/Aza vs Ven/Aza groups (Table 2). Magro/Aza achieved a composite CR (CRc: CR + CRi + CRh) rate of 12.9% vs 43.3% with Ven/Aza; the CR rate was 7.9% vs 30.8%. The median time to CR with Magro/Aza vs Ven/Aza was 2.55 vs 1.87 months; median duration of CR was 9.5 vs 4.6 months. A post hoc unplanned analysis among patients who received study treatment for >12 weeks (Magro/Aza, n = 33; Ven/Aza, n = 38) demonstrated similar differences in response rates to the ITT population, with ORRs of 57.6% and 78.9% and CRc rates of 36.4% and 73.7%, respectively (supplemental Table 2). Furthermore, 6-month OS rates among patients who received >12 weeks of treatment were 74.2% and 88.2%, respectively.
Response in the ITT population
| . | Eligible for nonintensive therapy . | Eligible for intensive therapy . | ||
|---|---|---|---|---|
| Magro/Aza (n = 101) . | Ven/Aza (n = 104) . | Magro/Aza (n = 27) . | 7+3 chemotherapy∗ (n = 25) . | |
| BOR, n (%) | ||||
| CR | 8 (7.9) | 32 (30.8) | 4 (14.8) | 7 (28.0) |
| CRMRD− | 1 (1.0) | 12 (11.5) | 0 | 2 (8.0) |
| CRi and CRh | 0 | 2 (1.9) | 0 | 0 |
| CRi only | 4 (4.0) | 9 (8.7) | 2 (7.4) | 3 (12.0) |
| CRh only | 1 (1.0) | 2 (1.9) | 0 | 1 (4.0) |
| MLFS | 5 (5.0) | 5 (4.8) | 0 | 1 (4.0) |
| PR | 6 (5.9) | 3 (2.9) | 0 | 1 (4.0) |
| SD | 49 (48.5) | 27 (26.0) | 17 (63.0) | 6 (24.0) |
| PD | 5 (5.0) | 4 (3.8) | 1 (3.7) | 2 (8.0) |
| No assessment | 23 (22.8) | 20 (19.2) | 3 (11.1) | 4 (16.0) |
| ORR (95% CI), % | 23.8 (15.9-33.3) | 51.0 (41.0-60.9) | 22.2 (8.6-42.3) | 52.0 (31.3-72.2) |
| CRc rate, % | 12.9 | 43.3 | 22.2 | 44.0 |
| . | Eligible for nonintensive therapy . | Eligible for intensive therapy . | ||
|---|---|---|---|---|
| Magro/Aza (n = 101) . | Ven/Aza (n = 104) . | Magro/Aza (n = 27) . | 7+3 chemotherapy∗ (n = 25) . | |
| BOR, n (%) | ||||
| CR | 8 (7.9) | 32 (30.8) | 4 (14.8) | 7 (28.0) |
| CRMRD− | 1 (1.0) | 12 (11.5) | 0 | 2 (8.0) |
| CRi and CRh | 0 | 2 (1.9) | 0 | 0 |
| CRi only | 4 (4.0) | 9 (8.7) | 2 (7.4) | 3 (12.0) |
| CRh only | 1 (1.0) | 2 (1.9) | 0 | 1 (4.0) |
| MLFS | 5 (5.0) | 5 (4.8) | 0 | 1 (4.0) |
| PR | 6 (5.9) | 3 (2.9) | 0 | 1 (4.0) |
| SD | 49 (48.5) | 27 (26.0) | 17 (63.0) | 6 (24.0) |
| PD | 5 (5.0) | 4 (3.8) | 1 (3.7) | 2 (8.0) |
| No assessment | 23 (22.8) | 20 (19.2) | 3 (11.1) | 4 (16.0) |
| ORR (95% CI), % | 23.8 (15.9-33.3) | 51.0 (41.0-60.9) | 22.2 (8.6-42.3) | 52.0 (31.3-72.2) |
| CRc rate, % | 12.9 | 43.3 | 22.2 | 44.0 |
BOR, best overall response; CRMRD−, CR without measurable residual disease; MLFS, morphologic leukemia-free state; SD, stable disease.
Seven days of continuous IV cytarabine (100 or 200 mg/m2 daily) and 3 days of IV daunorubicin (60 mg/m2 daily) or idarubicin (12 mg/m2 daily) on days 1 through 3.
Intensive arm
At final analysis, median OS in the intensive arm was 7.3 and 11.1 months with Magro/Aza and 7+3 chemotherapy, respectively (HR, 1.434; 95% CI, 0.635-3.239; P = .3798). Of 27 patients in the Magro/Aza group, 3 (11.1%) received SCT, and 8 of 25 (32.0%) patients in the 7+3 chemotherapy group received SCT.
ORRs were 22.2% vs 52.0% with Magro/Aza vs 7+3 chemotherapy (Table 2). Magro/Aza (n = 27) achieved a CRc rate of 22.2% vs 44.0% with 7+3 chemotherapy (n = 25); CR rates were 14.8% vs 28.0%.
Safety
The safety population included 244 patients who received ≥1 dose of study treatment.
Nonintensive arm
The median duration of exposure to Magro was 8.1 (range, 0.1-62.0) weeks and to Ven was 9.0 (range, 0.9-52.1) weeks. The most common reasons for study treatment discontinuation were PD (27.7%) for Magro/Aza and study termination (26.0%) for Ven/Aza (supplemental Table 3). The 30- and 60-day mortality rates after first dose of study drug were 10.4% and 21.9% (Magro/Aza) and 10.2% and 23.5% (Ven/Aza), respectively.
Magro/Aza safety was comparable with that of Ven/Aza (Table 3): grade ≥3 TEAEs occurred in 96.9% vs 95.9%, serious TEAEs in 87.5% vs 77.6%, and fatal TEAEs in 16.7% vs 19.4%, mainly driven by infections (6.3% vs 14.3%; supplemental Table 4). Among hematologic TEAEs, there were similar rates of grade ≥3 anemia (27.1% vs 23.5%), whereas the rate of grade ≥3 neutropenia was numerically lower with Magro/Aza vs Ven/Aza (17.7% vs 48.0%). Rates of grade ≥3 infections were similar between treatments (50.0% vs 53.1%). Rates of grade ≥3 infusion-related reaction were relatively low across both treatments (7.3% vs 1.0%). Change from baseline hemoglobin within the first week of treatment is shown in Figure 3. There was a median hemoglobin decrease of 1.8 g/dL from baseline 1 day after administration of the first dose of Magro vs a decrease of 0.1 g/dL with Ven/Aza. The median hemoglobin level approached baseline (−0.4 g/dL) 4 days after the first dose of Magro (and before the second dose), whereas in patients treated with Ven/Aza, hemoglobin was −0.8 g/dL relative to baseline hemoglobin 4 days after the first dose.
TEAEs occurring in 10% or more of patients in any treatment group eligible for nonintensive therapy
| TEAE, n (%) . | Magro/Aza (n = 96) . | Ven/Aza (n = 98) . | ||
|---|---|---|---|---|
| Any grade . | Grade ≥3 . | Any grade . | Grade ≥3 . | |
| Total | 96 (100.0) | 93 (96.9) | 97 (99.0) | 94 (95.9) |
| Related to any study drug | 78 (81.3) | 62 (64.6) | 80 (81.6) | 58 (59.2) |
| Febrile neutropenia | 46 (47.9) | 44 (45.8) | 48 (49.0) | 46 (46.9) |
| Pyrexia | 43 (44.8) | 4 (4.2) | 26 (26.5) | 5 (5.1) |
| Constipation | 33 (34.4) | 0 | 37 (37.8) | 0 |
| Anemia | 31 (32.3) | 26 (27.1) | 28 (28.6) | 23 (23.5) |
| Nausea | 28 (29.2) | 4 (4.2) | 32 (32.7) | 2 (2.0) |
| Diarrhea | 27 (28.1) | 1 (1.0) | 38 (38.8) | 0 |
| Thrombocytopenia∗ | 26 (27.1) | 23 (24.0) | 41 (41.8) | 35 (35.7) |
| Fatigue | 25 (26.0) | 4 (4.2) | 21 (21.4) | 6 (6.1) |
| IRR | 22 (22.9) | 7 (7.3) | 3 (3.1) | 1 (1.0) |
| Hypokalemia | 21 (21.9) | 4 (4.2) | 22 (22.4) | 9 (9.2) |
| Pneumonia | 21 (21.9) | 16 (16.7) | 18 (18.4) | 17 (17.3) |
| Decreased appetite | 20 (20.8) | 8 (8.3) | 21 (21.4) | 4 (4.1) |
| Dyspnea | 20 (20.8) | 4 (4.2) | 16 (16.3) | 3 (3.1) |
| Increased blood bilirubin | 19 (19.8) | 2 (2.1) | 11 (11.2) | 0 |
| Neutropenia† | 17 (17.7) | 17 (17.7) | 48 (49.0) | 47 (48.0) |
| Peripheral edema | 17 (17.7) | 1 (1.0) | 19 (19.4) | 0 |
| Vomiting | 15 (15.6) | 1 (1.0) | 22 (22.4) | 2 (2.0) |
| Asthenia | 15 (15.6) | 3 (3.1) | 13 (13.3) | 1 (1.0) |
| Headache | 13 (13.5) | 2 (2.1) | 11 (11.2) | 0 |
| Increased AST | 13 (13.5) | 2 (2.1) | 9 (9.2) | 1 (1.0) |
| Hypotension | 13 (13.5) | 3 (3.1) | 6 (6.1) | 2 (2.0) |
| Increased ALT | 13 (13.5) | 2 (2.1) | 6 (6.1) | 1 (1.0) |
| Hypophosphatemia | 12 (12.5) | 1 (1.0) | 8 (8.2) | 0 |
| Abdominal pain | 11 (11.5) | 2 (2.1) | 11 (11.2) | 2 (2.0) |
| Arthralgia | 11 (11.5) | 2 (2.1) | 10 (10.2) | 1 (1.0) |
| Hypomagnesemia | 11 (11.5) | 0 | 8 (8.2) | 0 |
| Pleural effusion | 11 (11.5) | 4 (4.2) | 6 (6.1) | 3 (3.1) |
| Hypoalbuminemia | 10 (10.4) | 1 (1.0) | 12 (12.2) | 1 (1.0) |
| Fall | 10 (10.4) | 1 (1.0) | 11 (11.2) | 1 (1.0) |
| Cough | 9 (9.4) | 0 | 10 (10.2) | 0 |
| Epistaxis | 8 (8.3) | 1 (1.0) | 15 (15.3) | 0 |
| Hyponatremia | 8 (8.3) | 1 (1.0) | 14 (14.3) | 3 (3.1) |
| Increase blood creatinine | 8 (8.3) | 1 (1.0) | 14 (14.3) | 1 (1.0) |
| Decreased WBC count | 6 (6.3) | 5 (5.2) | 14 (14.3) | 14 (14.3) |
| Increased blood ALP | 6 (6.3) | 1 (1.0) | 10 (10.2) | 0 |
| Injection site reaction | 5 (5.2) | 0 | 15 (15.3) | 0 |
| Stomatitis | 5 (5.2) | 0 | 15 (15.3) | 1 (1.0) |
| Decreased lymphocyte count | 4 (4.2) | 4 (4.2) | 11 (11.2) | 10 (10.2) |
| TEAE, n (%) . | Magro/Aza (n = 96) . | Ven/Aza (n = 98) . | ||
|---|---|---|---|---|
| Any grade . | Grade ≥3 . | Any grade . | Grade ≥3 . | |
| Total | 96 (100.0) | 93 (96.9) | 97 (99.0) | 94 (95.9) |
| Related to any study drug | 78 (81.3) | 62 (64.6) | 80 (81.6) | 58 (59.2) |
| Febrile neutropenia | 46 (47.9) | 44 (45.8) | 48 (49.0) | 46 (46.9) |
| Pyrexia | 43 (44.8) | 4 (4.2) | 26 (26.5) | 5 (5.1) |
| Constipation | 33 (34.4) | 0 | 37 (37.8) | 0 |
| Anemia | 31 (32.3) | 26 (27.1) | 28 (28.6) | 23 (23.5) |
| Nausea | 28 (29.2) | 4 (4.2) | 32 (32.7) | 2 (2.0) |
| Diarrhea | 27 (28.1) | 1 (1.0) | 38 (38.8) | 0 |
| Thrombocytopenia∗ | 26 (27.1) | 23 (24.0) | 41 (41.8) | 35 (35.7) |
| Fatigue | 25 (26.0) | 4 (4.2) | 21 (21.4) | 6 (6.1) |
| IRR | 22 (22.9) | 7 (7.3) | 3 (3.1) | 1 (1.0) |
| Hypokalemia | 21 (21.9) | 4 (4.2) | 22 (22.4) | 9 (9.2) |
| Pneumonia | 21 (21.9) | 16 (16.7) | 18 (18.4) | 17 (17.3) |
| Decreased appetite | 20 (20.8) | 8 (8.3) | 21 (21.4) | 4 (4.1) |
| Dyspnea | 20 (20.8) | 4 (4.2) | 16 (16.3) | 3 (3.1) |
| Increased blood bilirubin | 19 (19.8) | 2 (2.1) | 11 (11.2) | 0 |
| Neutropenia† | 17 (17.7) | 17 (17.7) | 48 (49.0) | 47 (48.0) |
| Peripheral edema | 17 (17.7) | 1 (1.0) | 19 (19.4) | 0 |
| Vomiting | 15 (15.6) | 1 (1.0) | 22 (22.4) | 2 (2.0) |
| Asthenia | 15 (15.6) | 3 (3.1) | 13 (13.3) | 1 (1.0) |
| Headache | 13 (13.5) | 2 (2.1) | 11 (11.2) | 0 |
| Increased AST | 13 (13.5) | 2 (2.1) | 9 (9.2) | 1 (1.0) |
| Hypotension | 13 (13.5) | 3 (3.1) | 6 (6.1) | 2 (2.0) |
| Increased ALT | 13 (13.5) | 2 (2.1) | 6 (6.1) | 1 (1.0) |
| Hypophosphatemia | 12 (12.5) | 1 (1.0) | 8 (8.2) | 0 |
| Abdominal pain | 11 (11.5) | 2 (2.1) | 11 (11.2) | 2 (2.0) |
| Arthralgia | 11 (11.5) | 2 (2.1) | 10 (10.2) | 1 (1.0) |
| Hypomagnesemia | 11 (11.5) | 0 | 8 (8.2) | 0 |
| Pleural effusion | 11 (11.5) | 4 (4.2) | 6 (6.1) | 3 (3.1) |
| Hypoalbuminemia | 10 (10.4) | 1 (1.0) | 12 (12.2) | 1 (1.0) |
| Fall | 10 (10.4) | 1 (1.0) | 11 (11.2) | 1 (1.0) |
| Cough | 9 (9.4) | 0 | 10 (10.2) | 0 |
| Epistaxis | 8 (8.3) | 1 (1.0) | 15 (15.3) | 0 |
| Hyponatremia | 8 (8.3) | 1 (1.0) | 14 (14.3) | 3 (3.1) |
| Increase blood creatinine | 8 (8.3) | 1 (1.0) | 14 (14.3) | 1 (1.0) |
| Decreased WBC count | 6 (6.3) | 5 (5.2) | 14 (14.3) | 14 (14.3) |
| Increased blood ALP | 6 (6.3) | 1 (1.0) | 10 (10.2) | 0 |
| Injection site reaction | 5 (5.2) | 0 | 15 (15.3) | 0 |
| Stomatitis | 5 (5.2) | 0 | 15 (15.3) | 1 (1.0) |
| Decreased lymphocyte count | 4 (4.2) | 4 (4.2) | 11 (11.2) | 10 (10.2) |
ALP, alkaline phosphatase; ALT, alanine aminotransferase; AST, aspartate aminotransferase; IRR, infusion-related reaction; WBC, white blood cell.
Includes preferred terms of thrombocytopenia and decreased platelet count.
Includes preferred terms of neutropenia and decreased neutrophil count.
Change from baseline in hemoglobin in the first week after infusion for each treatment group in the nonintensive therapy arm. Medians ± interquartile range are shown. Only 1 patient received Ven/Aza on day 1+. D, day; WK, week.
Change from baseline in hemoglobin in the first week after infusion for each treatment group in the nonintensive therapy arm. Medians ± interquartile range are shown. Only 1 patient received Ven/Aza on day 1+. D, day; WK, week.
Intensive arm
The median duration of exposure to Magro was 8.6 (range, 0.1-68.1) weeks. The majority of the 7+3 chemotherapy group received 1 induction cycle (n/N, 20/23 [87.0%]) and no consolidation cycles (n/N, 15/23 [65.2%]); no patient received >2 induction or consolidation cycles. Two (9%) patients underwent reinduction therapy in the 7+3 chemotherapy group. The most common reason for study treatment discontinuation was study termination (25.9%) with Magro/Aza and SCT (24.0%) with 7+3 chemotherapy (supplemental Table 3). The 30- and 60-day mortality rates after the first dose of study drug were 7.4% and 22.2%, respectively, for Magro/Aza, and 8.7% and 8.7%, respectively, for 7+3 chemotherapy.
Magro/Aza safety was similar to that of 7+3 chemotherapy (supplemental Table 5): grade ≥3 TEAEs occurred in 92.6% vs 95.7%, serious TEAEs presented in 74.1% vs 60.9%, and fatal TEAEs in 18.5% vs 8.7% (supplemental Table 4). Hematologic grade ≥3 TEAEs of anemia and neutropenia were similar between Magro/Aza vs 7+3 chemotherapy (25.9% vs 21.7% and 11.1% vs 13.0%, respectively). Rates of grade ≥3 infectious complications with Magro/Aza vs 7+3 chemotherapy, including sepsis (14.8% vs 21.7%) and pneumonia (11.1% vs 13.0%), were numerically lower (44.4% vs 65.2%). Rates of grade ≥3 infusion-related reaction was low across both treatment groups (3.7% vs 0%).
Discussion
ENHANCE-2 is, to our knowledge, the first randomized, phase 3 study in TP53-mutated AML, for which outcomes are particularly poor and new treatment options are urgently needed.8 An interim futility analysis for the study determined that the futility boundary was passed for the primary analysis population of patients eligible for nonintensive therapy; thus, the study was terminated. Although there was no futility boundary for patients eligible for intensive therapy, OS outcomes were similar among this population (HR, 1.434 [95% CI, 0.635-3.239]) at the same final analysis. Similar to OS, other efficacy end points showed a trend for worse outcomes in the Magro/Aza groups vs the respective controls, and ORRs were lower with Magro/Aza vs either control. Additionally, although overall rates of SCT in this study population were low, the proportion of patients proceeding to SCT was lower in both Magro/Aza groups compared with the respective controls.
ENHANCE-2 was designed based on results from a previous phase 1b (ClinicalTrials.gov identifier: NCT03248479) study of Magro/Aza for patients with previously untreated AML who were ineligible for IC.15 Ultimately, the Magro/Aza results were not recapitulated in ENHANCE-2 when compared with results from the phase 1b study of Magro/Aza (median OS, 4.4 vs 9.8 months; CRc rate, 12.9% vs 40.3%; ORR, 23.8% vs 47.2%).15 In contrast, Ven/Aza results in ENHANCE-2 were consistent with those previously reported for the pooled analysis of treatment-naïve patients with TP53-mutated AML treated with Ven/Aza (including in VIALE-A), wherein the CRc rate (defined as CR + CRi2) and median OS were 47.6% and 5.5 months, respectively.16
There are several potential explanations why Magro/Aza was found to be less effective in ENHANCE-2 than in the prior phase 1b study.15 Importantly, herein, because of the futility determined at interim analysis, median duration of Magro exposure in both nonintensive and intensive arms (median, ≈8 weeks) was substantially shorter than intended in the study design and compared with that administered in the previous phase 1b study (median, ≈12 weeks).15 In the phase 1b study of Magro/Aza, median time to CR was 3.7 months, and 26% of patients who achieved a CR did so after 4 months of Magro/Aza.15 An exploratory post hoc analysis of ENHANCE-2 demonstrated that only 33 of 101 patients in the nonintensive arm randomized to Magro/Aza received ≥12 weeks of treatment (supplemental Table 2). Among this minority, the CRc rate and ORR were 36.4% and 57.6%, respectively, which was more consistent than the ITT analysis with the overall results in the phase 1b study of Magro/Aza.15 Thus, it is possible that that overall responses and clinical outcomes could have improved with sufficient exposure to Magro/Aza. However, early disease progression, intolerability, and early death, which were all more common with Magro/Aza in ENHANCE-2 compared with the prior phase 1b study15 (eg, death within 30 days: 10.4% vs 6.9%; death within 60 days: 21.9% vs 16.1%), likely contributed to the low proportion of patients receiving sufficient exposure to Magro/Aza.
More rapid responses were achieved with Ven/Aza (median time to CR, 1.87 months) than with Magro/AZA in ENHANCE-2, and median time to CR for Ven/Aza was consistent with results reported in VIALE-A (median time to CR/CRi, 1.3 months).2 This difference in time to CR between Magro/Aza and Ven/Aza may have contributed to early discontinuation of Magro/Aza, leading to worse clinical outcomes. In ENHANCE-2, response to 7+3 chemotherapy also occurred within 2 months without the need for ongoing continuous therapy as is necessary for Magro/Aza treatment.
The low response rates seen with Magro/Aza likely explain the lack of OS benefit in ENHANCE-2. For example, SCT is a key driver of OS, and SCT rates were lower for Magro/Aza in ENHANCE-2 when compared with the phase 1b study15 and for Magro/Aza vs Ven/Aza in ENHANCE-2, potentially because of lower response rates. However, the number of patients who proceeded to SCT in ENHANCE-2 was very small (11 patients across intensive-arm groups), precluding any informative subgroup analyses of transplanted patients and preventing any definitive conclusions from being drawn. Interestingly, the nonintensive-arm OS appeared comparable between Magro/Aza and Ven/Aza within the first 4 months on study, with the curve separation thereafter (Figure 2). Among the minority of patients who did not have an early EFS event, outcomes appear similar between Magro/Aza and Ven/Aza (supplemental Figure 3). Among those who achieved CR, median duration of CR was numerically longer with Magro/Aza vs Ven/Aza (9.5 vs 4.6 months). Ultimately, lower response rates and less effective early disease control correlated with poorer survival in Magro-treated patients.
Lastly, although the proportion of patients with monoallelic vs biallelic TP53 mutations was unknown in the phase 1b study of Magro/Aza,15 patients in ENHANCE-2 carried predominantly biallelic TP53 mutations, which has been shown to result in worse outcomes compared with monoallelic TP53 mutations.18 It is possible that the clinical activity of Magro/Aza may be different in patients with monoallelic vs biallelic TP53 mutations.19
The safety profiles of both Magro/Aza groups were similar to those in the respective control groups, with a similar number of fatal AEs. Although the cumulative Magro/Aza safety profile was characterized previously, comparison with Ven/Aza remains difficult because the mechanisms of Magro’s effects on myelosuppression and infection risk are different from that of Ven and may not correlate in the same manner. For example, there were similar rates of febrile neutropenia and high-grade infections between nonintensive-arm treatment groups, despite lower rates of neutropenia with Magro/Aza. Thus, a correlation between neutropenia and infections, previously observed in patients with AML, was not as evident with Magro/Aza.
Anemia is an expected on-target effect of Magro after the first 1 to 2 doses. In ENHANCE-2, the overall frequency of grade ≥3 anemia was similar across both therapeutic arms, and the frequencies reported in both Magro/Aza groups were consistent with that in the phase 1b study of Magro/Aza.15 In ENHANCE-2, a hemoglobin requirement of ≥9 g/dL before the first 2 Magro doses and recheck of levels 3 to 6 hours after these infusions combined with a priming/maintenance dose strategy were used to mitigate anemia in cycle 1. Hemoglobin levels decreased by a median of 1.8 g/dL from baseline after the first dose of Magro and increased before the second dose. By the end of week 1, many patients had increased hemoglobin levels compared with levels after the first dose, consistent with previous observations.15
To date, the only other investigational agent with reported data in TP53-mutated myelodysplastic syndromes/AML is the P53 reactivator eprenetapopt. In phase 1/2 studies, eprenetapopt plus Aza showed promising activity as first-line treatment or as post-SCT maintenance.20 However, a randomized phase 3 study of this combination in TP53-mutated myelodysplastic syndromes did not demonstrate improved CR rates vs Aza alone.21
Optimal management of patients with TP53-mutated AML remains an immense challenge in AML that is not addressed by the current treatment armamentarium. Patients with AML are frequently older and ineligible for IC. Although the randomized phase 3 VIALE-A trial showed that Ven/Aza improved OS in patients with previously untreated AML,2 subset analyses in TP53-mutated AML, including patients from VIALE-A, found no differences in OS between patients who received Ven/Aza compared with those who received placebo/Aza.16 Findings from ENHANCE-2 underscore the challenges of developing effective therapies in this extremely difficult-to-treat population and provide important information regarding the activity and clinical outcomes with Ven/Aza in a prospective randomized international study. ENHANCE-2 is, to our knowledge, the first large, randomized study to evaluate this combination in this population specifically. Although Magro/Aza did not lead to improved OS in TP53-mutated AML, our findings also validate that Ven/Aza outcomes are unsatisfactory (median OS, 6.6 months). These data reinforce the continued poor outcomes with conventional therapies in TP53-mutated AML and provide a foundation and impetus to explore novel therapies and combinations in this patient population.
Acknowledgments
The authors thank the patients and their caregivers and families for their participation and commitment to this clinical study. Medical writing and editorial assistance was provided by Miranda Bader-Goodman and Ebenezer M. Awuah-Yeboah of Ashfield MedComms, an Inizio company, and was funded by Gilead Sciences, Inc.
Authorship
Contribution: L.J., M.D., T.B., C.L., J.F.Z., D.A.S., and P.V. contributed to study conception and design; J.F.Z., D.A.S., C.R., N.G.D., A.Y.H.L., D.K.H., M.S., T.P., P.M., R.A.L., L.W., A.K.E., I.K., C.P., J.O., and P.V. contributed to provision of study materials or patients; L.J., M.D., J.H., T.B., G.H.K., and C.L. collected and assembled data; J.H. performed statistical analysis; and all authors read, provided critical revisions, and approved the manuscript.
Conflicts-of-interest disclosure: J.F.Z. reports consulting or advisory roles for AbbVie, AstraZeneca, Daiichi Sankyo, Foghorn, Genmab, Gilead, NeoGenomics, Novartis, Sellas, Servier, Shattuck Labs, Sumitomo Dainippon Pharma, and Syndax; and reports research funding from AbbVie, Akesobio, Arog, AstraZeneca, Faron, Gilead, Jazz Pharmaceuticals, Loxo, Merck, Newave, Novartis, Sellas, Shattuck Labs, Stemline, Sumitomo Dainippon Pharma, and Zentalis. D.A.S. reports consulting or advisory roles for AbbVie, Affimed, Agios, Amgen, Aprea, Arog, Astellas Pharma, AvenCell, Bluebird Bio, Bristol Myers Squibb (BMS)/Celgene, Daiichi Sankyo, Genentech, Gilead Sciences, Incyte, Immunogen, Intellia, IntelliSphere, Jasper Therapeutics, Jazz Pharmaceuticals, Kite, Magenta Therapeutics, Molecular Partners AG, Nemucore, NKarta, Novartis, Orbital Therapeutics, Pfizer, PGEN Therapeutics, Rigel Pharmaceuticals, Servier, Shattuck Labs, Stemline/Menarini, Syndax, Syros, Takeda, Trillium Therapeutics, and Zentalis; and research funding to his institute from AbbVie, Amgen, Aprea, Astellas Pharma, BMS, Daiichi Sankyo, Fate Therapeutics, Genentech, Gilead Sciences, Glycomimetics, Hanmi, Immunogen, Kite, Novimmune, Pfizer, Servier, Syntrix Pharmaceuticals, Trillium Therapeutics, and Trovagene; and reports patents and royalties from Lixte. C.R. reports consulting or advisory roles for AbbVie, Amgen, Astellas, BMS, Boehringer, Daiichi Sankyo, Jazz Pharmaceuticals, Janssen, and Servier; received research funding from AbbVie, Amgen, Astellas, BMS, Daiichi Sankyo, IQVIA, and Jazz Pharmaceuticals; and received support for attending meetings and/or travel from AbbVie, Novartis, and Servier. N.G.D. reports consulting roles for AbbVie, Agios, Amgen, Arog, Astellas, BMS/Celgene, Daiichi Sankyo, Genentech, Gilead, ImmunoGen, Jazz Pharmaceuticals, Kite, Novartis, Pfizer, Servier, Shattuck Labs, Stemline/Menarini, Syndax, and Trillium; and reports research funding to his institution from AbbVie, Amgen, Astellas, BMS, Daiichi Sankyo, Fate Therapeutics, Genentech, Gilead, Glycomimetics, Hanmi, ImmunoGen, Kite, Novimmune, Pfizer, Servier, Trillium, and Trovagene. D.K.H. reports advisory roles for AbbVie and Otsuka. M.S. reports consulting or advisory roles for Autolus, AvenCell, CanCell Therapeutics, CDR-Life, Genmab US, Ichnos Sciences, Incyte Biosciences, Interius BioTherapeutics, Janssen, Millennium Pharmaceuticals, Miltenyi Biomedicine, Molecular Partners, Nektar Therapeutics, Novartis, Pfizer, Ridgeline Discovery, Sanofi, Scare, and Takeda; serves on speakers' bureau for Amgen, AstraZeneca, BMS/Celgene, Gilead/Kite, GSK, Janssen, Novartis, Octapharma, Pfizer, Roche, Springer Healthcare, and Takeda; received research funding from Amgen, BMS/Celgene, Gilead/Kite, Janssen, Miltenyi Biotec, Molecular Partners, MorphoSys, Novartis, Roche, Seattle Genetics, and Takeda; and received educational grants to develop the application “MyTcell” from BMS. P.M. reports consulting roles for AbbVie, Astellas, BeiGene, BMS, Gilead, Incyte, Jazz Pharmaceuticals, Kura Oncology, Menarini/Stemline, Nerviano, Novartis, Otsuka, Pfizer, Ryvu, and Takeda; reports research funding from AbbVie, BMS, Jazz Pharmaceuticals, Menarini/Stemline, Novartis, Pfizer, and Takeda; and served on speakers' bureaus for AbbVie, Astellas, BMS, Gilead, Jazz Pharmaceuticals, and Pfizer. R.A.L. reports consulting or advisory roles for AbbVie, Actinium Pharmaceuticals, Amgen, Ariad/Takeda, Astellas, BMS/Celgene, Curis, CVS Caremark, Epizyme, Immunogen, Jazz Pharmaceuticals, Kling Biotherapeutics, MedPace, MorphoSys, Novartis, Rigel Pharmaceuticals, Servier, and Takeda Science Foundation; reports research funding to his institution from Astellas, Celgene, Cellectis, Daiichi Sankyo, Gilead/Forty Seven, Novartis, and Rafael Pharmaceuticals; and reports royalties from UpToDate Inc. A.K.E. reports advisory roles and speakers' bureau participation for ASTEX/Otsuka. C.P. reports; advisory roles for AbbVie, Astellas, Blueprint Medicine, Delbert Laboratories, GSK, Istituto Gentili, Janssen, Jazz Pharmaceuticals, Novartis, Pfizer, and Syndax; and reports honoraria from AbbVie, Amgen, Astellas, BMS, Incyte, Istituto Gentili, Janssen, Menarini/Stemline, Pfizer, Novartis, and Servier. J.O. reports advisory roles for AbbVie; and reports travel/accommodation or honoraria from AbbVie, Astellas, Janssen, Jazz Pharmaceuticals, and Servier. L.J., M.D., J.H., T.B., G.H.K., and C.L. are employed by, and hold stock in, Gilead Sciences. P.V. reports advisory roles or travel/accommodation from AbbVie, Auron Therapeutics, ImmunoGen, Jazz Pharmaceuticals, Kura, Pfizer, Rigel, Servier, Stemline/Menarini, and Takeda; reports honoraria from AbbVie, Astellas, Celgene, Daiichi Sankyo, Jazz Pharmaceuticals, and Pfizer; reports speakers' bureau participation with AbbVie, Astellas, Gilead, and Servier; reports research funding to his institution from BMS/Celgene; reports stock or ownership in Auron Therapeutics and Yellowstone Biosciences; and reports patents for flow cytometric detection of leukemic stem cells. The remaining authors declare no competing financial interests.
A complete list of the ENHANCE-2 investigators appears in the supplemental Appendix.
Correspondence: Joshua F. Zeidner, Lineberger Comprehensive Cancer Center, The University of North Carolina at Chapel Hill, Houpt Building, 3rd Floor, 170 Manning Dr, CB #7305, Chapel Hill, NC 27599; email: joshua_zeidner@med.unc.edu.
References
Author notes
Gilead Sciences shares anonymized individual patient data upon request or as required by law or regulation with qualified external researchers on the basis of submitted curriculum vitae and reflecting no conflict of interest. The request proposal must also include a statistician. Approval of such requests is at Gilead Science’s discretion and is dependent on the nature of the request, the merit of the research proposed, the availability of the data, and the intended use of the data. Data requests should be sent to datarequest@gilead.com.
The online version of this article contains a data supplement.
There is a Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal