In this issue of Blood, Shah et al found that resistance to BRD4/BET inhibition in acute myeloid leukemia (AML) could be attenuated with properly timed inhibition of the p300 coactivator.1
Over a decade ago, the bromodomain and extraterminal (BET) protein BRD4 was identified as a therapeutic target in AML, a disease that is often driven by dysregulated epigenetic and transcriptional regulation.2,3 A large body of mechanistic research supports a model in which BRD4-containing protein complexes maintain transcription of AML-promoting genes (eg, MYC) via the attachment of BRD4 bromodomains with acetylated histones and transcription factors at enhancer and superenhancer elements.4 Owing to this mechanism, pharmacological targeting of the BRD4 bromodomains extends the survival of AML-bearing mice, with evidence of therapeutic index in multiple genetic subtypes of this heterogeneous disease. Nonetheless, it was evident during preclinical investigation that BRD4/BET inhibition alone was insufficient to produce regressions in animal models, owing in part to toxicities that limit the extent to which BRD4/BET can be suppressed in vivo. In addition, laboratory studies revealed that AML cells are adept at nongenetically reprogramming their transcriptome to adapt to chronic BRD4 inhibition.5,6 Clinical investigation would later validate these observations in human patients with AML: therapeutic responses to BRD4/BET inhibitor monotherapies were found to be variable and lacked durability, with toxicities (eg, thrombocytopenia) requiring noncontinuous drug dosing, which swiftly selects for drug-resistant cell populations.7,8
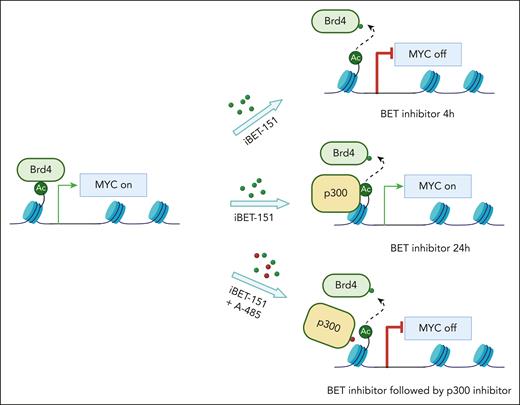
Although BRD4, like many other epigenetic regulators, has inherent limitations as a cancer target, one path forward has been to explore combination regimens that magnify the anti-AML effects of BRD4/BET inhibitors, but without exacerbating toxicities. Indeed, preclinical studies and a phase III clinical trial suggest that BRD4/BET inhibitors can be combined with JAK2 inhibitors in myeloproliferative disease to enhance therapeutic responses.9,10 Whether a similarly efficacious combination regimen can be identified in AML is an important question in the field and the subject investigated in the study by Shah et al. Using an analysis of acute epigenomic effects of BRD4/BET inhibition, they identified a compensatory feedback response to these inhibitors, in accord with prior findings,5,6 in which gene expression changes become attenuated in a time-dependent manner. They identified a causal role of the lysine acetyltransferase coactivator protein p300 in this process, which became redistributed to sites of BRD4 eviction. The functional consequence of this observation was that coadministration of BRD4/BET inhibitors and p300 inhibitors disrupted the compensatory feedback response and led to sustained transcriptional inhibition of key AML-promoting genes (eg, MYC) (see figure).
Combination of BET and p300 inhibitors mitigated BET inhibitor resistance by suppressing the expression of genes critical for AML maintenance.
Combination of BET and p300 inhibitors mitigated BET inhibitor resistance by suppressing the expression of genes critical for AML maintenance.
The authors then went on to explore the preclinical implications of this mechanism by combining inhibitors of BRD4/BET and p300 in various dosing and timing schedules. The main takeaway from these experiments was that sequential dosing was found to be most synergistic, with BRD4/BET inhibition first followed by p300 inhibition to attenuate the compensatory feedback response. In addition, they evaluated a sample collected from a patient with AML from a recent clinical trial, to validate that following patient treatment with a BRD4/BET inhibitor the leukemic cells became hypersensitive to p300 inhibition. These results reveal a window of opportunity in which a properly timed delivery of p300 inhibition after BRD4/BET inhibition can lead to deeper anti-AML effects, but seemingly without a more severe impairment of normal hematopoiesis.
This important study builds upon prior findings by identifying an actionable target and a dosing strategy for avoiding the acute transcriptional compensation that accompanies BRD4/BET inhibition. Although p300-targeting drugs have yet to reach the clinic, active drug development programs exist for this target in the biotech industry, and hence an opportunity exists for the findings in this study to reach the clinic. One area of concern for translating these findings is regarding the tolerability of combining drugs that target 2 general transcriptional coactivator proteins at the same time, particularly since the tolerability of BRD4/BET inhibition alone poses obstacles in the clinic. However, an interesting concept introduced by Shah et al is that timing might be everything when targeting these 2 coactivators. Perhaps a pulsatile sequential delivery regimen of BRD4/BET and p300 inhibition might deliver an irreversible arrest in AML proliferation, whereas normal hematopoietic cells might be able to recover from these 2 drugs. In addition, this work further highlights how a time-resolved epigenomic analysis of acute drug effects can lead to insights into how to build combination epigenetic therapies, a concept that remains of broad significance to cancer biology.
Conflict-of-interest disclosure: The authors declare no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal