In this issue of Blood, Sehn and colleagues provide long-term follow-up on treatment with fixed-duration mosunetuzumab, a CD20×CD3 bispecific antibody (BsAb), in patients with relapsed or refractory (R/R) follicular lymphoma (FL). Results from the phase 2 portion of the pivotal phase 1 to 2 dose-escalation trial (NCT02500407) demonstrate high rates of durable responses with limited toxicity in a high-risk patient population.1
R/R FL is considered incurable with diminishing returns with each subsequent line of therapy.2 Outcomes are particularly poor in patients with progression of disease within 24 months of initial therapy (POD24) or multiply relapsed disease and those harboring high-risk mutations such as TP53.2-4
In the last 2 to 3 years, the treatment landscape for R/R FL has evolved rapidly with several agents recently approved by the US Food and Drug Administration and the European Medicines Agency. These include the Bruton’s tyrosine kinase inhibitor, zanubrutinib in combination with the anti-CD20 monoclonal antibody obinutuzumab, the EZH2 inhibitor tazemetostat, and chimeric antigen receptor T-cell (CART) constructs axicabtagene ciloleucel (axi-cel) and lisocabtagene maraleucel (liso-cel).5-8
In addition, 3 CD20×CD3 BsAbs have been approved in the United States or in Europe for the treatment of R/R FL after ≥2 prior systemic therapies and function by redirecting T-cell cytotoxicity toward malignant B cells. These include epcoritamab, odronextamab, and mosunetuzumab. Pivotal trials for all 3 agents enrolled heavily pretreated patients with similar incidence of high-risk subsets such as those refractory to both prior anti-CD20 and alkylator therapy or POD24. Although not directly comparable, early response rates and durability of responses seem to be similar for these agents, accounting for differences in median follow-up. Sehn and colleagues, however, provide the longest follow-up data for a BsAb in R/R FL and further insights on outcomes of mosunetuzumab therapy according to mutational status.9,10
To date, a tempered enthusiasm for BsAb treatment of R/R FL has been fueled by relatively short-term follow-up and the evolving treatment landscape with various alternative therapeutic options available, some with potential for cure. Sehn and colleagues address these concerns in their 3-year update for their phase 2 data evaluating mosunetuzumab in R/R FL, providing the longest follow-up for a BsAb in this patient population.
In this study, patients received intravenous mosunetuzumab in 21-day cycles with step-up dosing in cycle 1 to mitigate immune-mediated toxicities. Patients achieving a complete response (CR) by cycle 8 stopped treatment at this time point, and patients with a partial response or stable disease continued to a total of 17 cycles.
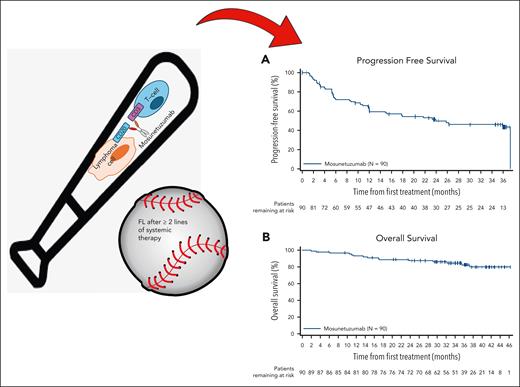
At a median follow-up of 37.4 months, mosunetuzumab produced a durable CR rate of 60% (median duration of CR was not reached) as fixed-duration treatment, with most patients receiving ≤8 cycles. Estimated 36-month progression-free survival (PFS) and overall survival (OS) rates were 43% and 82%, respectively (see figure). Cytokine release syndrome (CRS) events occurred in 44% of patients and were grade 3 to 4 in severity in 2%, primarily with the first cycle. No new safety signals were observed with longer follow-up.
Mosunetuzumab, a CD20×CD3 BsAb, demonstrates activity in heavily pretreated FL including those with high-risk features and provides encouraging survival outcomes with Kaplan-Meier estimates for PFS (A) and OS (B) in the intent to treat population (n = 90).
Mosunetuzumab, a CD20×CD3 BsAb, demonstrates activity in heavily pretreated FL including those with high-risk features and provides encouraging survival outcomes with Kaplan-Meier estimates for PFS (A) and OS (B) in the intent to treat population (n = 90).
Several high-risk subsets were included among a total of 90 patients on trial: two-thirds were refractory to last prior therapy, half were refractory to prior anti-CD20 and alkylator therapy or progressed within 24 months, and one-fifth had TP53 mutations. Mosunetuzumab demonstrated encouraging results in each of these high-risk subsets.1
The CART trials ZUMA-5 (for axi-cel) and TRANSCEND (for liso-cel) demonstrated CR rates of 94% and 79%, respectively, and also included heavily pretreated patients and other high-risk subsets.7,8 Recently, updated results from ZUMA-5 with over 3 years of follow-up were reported, comparable in duration to this phase 2 trial of mosunetuzumab. The 3-year PFS and OS rates with axi-cel were 54% and 75%, respectively, with evidence of an emerging plateau in PFS beyond 2 years. Similar outcomes are reported for mosunetuzumab in this trial for complete responders.1
As such, how should we position our robust batter against the formidable competing team of CART constructs?
Things to consider include prior bendamustine exposure. For patients requiring therapy within 1 year of prior bendamustine exposure, outcomes with CART are less compelling. As for toxicity, CRS events observed with the 41BB construct liso-cel and mosunetuzumab are typically low grade and manageable. However, one should keep in mind that although most CRS events occur in cycle 1 with mosunetuzumab, CRS was reported in 10% after C2D1 and 2% in any subsequent cycle.
An unresolved question is how best to sequence these T-cell-engaging treatments. In this study, few patients received prior CART treatment. Data for BsAb either before CART or post-CART failure remain limited. Compared with CART, the advantages of mosunetuzumab are quick access to an off-the-shelf treatment, outpatient administration, low-grade and predictable CRS, and low incidence of immune-effector cell-associated neurologic syndrome. These factors may place it ahead of CART for some patients. Alternatively, if there is high suspicion for transformed disease, CART may be favored here with its established track record in transformations.
Through exploratory analyses, additional noteworthy data with practical implications for the clinician pertaining to treatment with mosunetuzumab are as follows: (1) partial responses were relatively short-lived, lasting less than 12 months, and one should be prepared to pivot quickly to an alternative therapy in these patients; (2) median time to CD19+ B-cell recovery was 18.4 months in complete responders following 8 cycles of mosunetuzumab, an advantage of fixed-duration treatment that should guide evaluation for hypogammaglobulinemia and prophylactic antimicrobial therapy; (3) lastly, patients may be retreated with mosunetuzumab to reharness a response in some cases. As BsAbs make their way to earlier lines of therapy, the questions of sequencing will evolve. Sehn and colleagues provide signals that retreatment with mosunetuzumab may still be an option in these cases.
For patients with R/R FL including those with high-risk features, long-term data provided by Sehn and colleagues underscore the capacity of BsAbs to mitigate high-risk biology by providing responses more durable than expected historically. The identification of biomarkers of response will be key. These data support the ongoing investigations of BsAbs in combination with other agents in earlier lines of therapy with the potential to circumvent early relapse or progression and foster a major shift in treatment paradigms for FL.
Conflict-of-interest disclosure: J.N.W. declares research funding and honoraria from Merck and honoraria from Genentech and Bristol Myers Squibb (BMS). R.K. declares advisory board fees from AstraZeneca, BMS, Gilead Sciences/Kite Pharma, Janssen, Pharmacyclics, Morphosys/Incyte, Genentech/Roche, and AbbVie; grants/research support from BMS, Takeda, BeiGene, Gilead Sciences/Kite, and Calithera; and speaker’s bureau fees from BeiGene, AstraZeneca, and Morphosys/Incyte.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal