In this issue of Blood, Darbinian et al1 report that the interaction between the genotype and a colitogenic microbiome elevates inflammasome activation within the intestinal barrier, and regulatory T-cell (Treg) numbers and mucin production diminish. This detrimental interaction exacerbates the severity of colitis in the gp91phox−/− mouse model of chronic granulomatous disease (CGD).
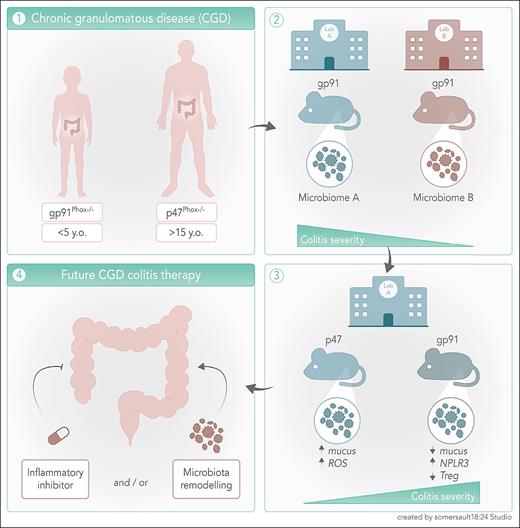
CGD is a primary immunodeficiency caused by mutations in one of the subunits of the reduced NAD phosphate (NADPH) oxidase complex 2 (NOX2). Mutations in the CYBA/P22PHOX, CYBB/GP91PHOX, NCF1/P47PHOX, NCF2/P67PHOX, and NCF4/P40PHOX severely compromise NOX2-dependent production of reactive oxygen species (ROS).2 Without an intact oxidative burst, phagocytes from patients with CGD cannot effectively clear pathogens, especially catalase-positive bacteria and fungi. Additionally, teenagers and young adults with CGD are at greater risk of developing chronic (auto)inflammatory diseases, with 50% suffering from Crohn colitis–like inflammatory bowel disease (IBD).3,4
IBD appears at younger age and is particularly severe in patients with CGD carrying the X-linked CYBB mutation, whereas patients carrying autosomal recessive mutations, like NCF1, develop a less aggressive disease in their late teens or early adulthood.4 Interestingly, patients with CGD with either active IBD or a history of IBD have distinct microbiomes and microbiota metabolomes from those without IBD.5 The role of gastrointestinal microbiota in shaping the events of intestinal diseases has long been recognized. Unfortunately, cause and correlation are frequently misinterpreted, and thus the proof of a true causal relationships between dysbiosis and IBD has not been definitively established.6 However, it has been shown that a gastrointestinal microbiome signature is associated with a future development of Crohn disease (CD), thus demonstrating that dysbiosis precedes CD onset by up to 5 years.7 This established a true causal relationship and paved the way for a broader exploration of the microbiota role in intestinal diseases onset and progression. Nonetheless, in CGD there is still a need for a more robust evaluation of the inflammatory mechanism(s) behind the genotype differences in IBD development and how they influence the composition of the intestinal microbiota.
Darbinian et al try to close this knowledge gap by inducing acute dextran sulfate sodium colitis in wild-type (WT), gp91phox−/−, and p47phox−/− mice housed in 2 different animal facilities (National Institutes of Health [NIH] and Institut de Recherches Cliniques de Montréal [IRCM]) and combining microbiomic, transcriptomic, and functional analysis. They observed that colitis in p47phox−/− mice from both facilities and IRCM gp91phox−/− mice was more severe than in WT mice, whereas disease severity in NIH gp91phox−/− mice was comparable to WT. Through 16S ribosomal RNA gene sequencing on DNA extracted from mouse stools in both facilities, the authors proved that different genotypes carried distinct microbiota and that the animal facilities also contributed to (colitogenic) microbiome diversity between genotypes. Using elegant cross-fostering experiments, the authors increased colitis severity in NIH gp91phox−/− mice, which demonstrates that early life exposure to colitogenic microbiota leads to the development of severe colitis in less susceptible strains. Importantly, the authors were able to identify several microbiome-associated metabolic pathways that were common between mice in both facilities and patients with CGD, which underlines the pertinence of these findings to understand the development and mechanisms of IBD in CGD.
In the normal interactions between microbiome and the intestinal epithelium, ROS is produced by homologues of the NOX2 only to such an extent that they do not damage the integrity of the intestinal barrier. To show that in the presence of dysbiosis ROS production is abnormal and may compromise the integrity of the barrier, the authors sorted colon epithelial cells (CECs) from mice in the same animal facility and demonstrated that those from colitis-susceptible p47phox−/− mice produced more superoxide and had a higher expression of the NOX2 homologue NOX1, whereas gp91phox−/− mice did not exhibit any of these changes. RNA sequencing analysis of CECs sorted before and after colitis development revealed that the mechanisms behind colitis severity in gp91phox−/− mice are distinct from those in p47phox−/− mice. At baseline the 2 strains presented differences in the expression of genes involved in pathways related to immune system regulation and inflammatory response. Colitis in ph47phox−/− mice induced the expression of genes coding for proteins involved in mucus production, which was confirmed by a thicker mucus layer and more intestinal mucus-producing goblet cells. The transcriptome in gp91phox−/− mice after colitis induction was characterized by upregulated genes coding for proteins related to major histocompatibility complex, interferon stimulation, and inflammasome activation. To determine the impact of the colitis-induced gene expression changes in the composition of the intestinal immune cell compartment, the authors quantified the frequencies of antibody-secreting B cells (ASCs), T-cell subsets, and cytokine-producing myeloid cells in the intestinal lamina propria. After colitis induction more ASC, and less regulatory T lymphocytes (Treg) and interleukin 10 (IL-10)-producing (anti-inflammatory) myeloid cells, were observed in gp91phox−/− mice.
Darbinian et al clearly demonstrate that compared with p47phox−/− mice, the intestinal barrier from gp91phox−/− mice respond to colitogenic microbiota by reducing the production of mucin and ROS, by lowering the number of anti-inflammatory leukocytes, and by enhancing inflammasome activation (see figure). The study results suggest that inflammasome-blockade and microbiome remodeling could have positive effects in colitis management in CGD. Inflammasome inactivation through IL-1β-blockade has shown promising results in children with IBD8 and may have the potential to improve IBD in the very young patients with CYBB-mutant CGD. Dietary changes, like more consumption of probiotics and reducing ultraprocessed foods, are increasingly being explored as a means of changing the gastrointestinal microbiome and treating IBD. Strategies either focused on resolving dysbiosis or providing precise products of microbiome metabolism9,10 may prove effective in curbing IBD in CGD. These approaches may break the colitis-promoting genotype-derived interactions between the intestinal barrier and microbiota and foster the development of tailored therapies for patients with CGD.
Patients carrying the X-linked CYBB/GP91PHOX mutation of NOX2 develop a more severe IBD at an earlier age than those carrying the autosomal NCF1/P47PHOX mutation. The genotype shapes the inflammatory response against colitogenic microbiota and determines the severity of colitis, by altering mucus production, activating the inflammasome, and reducing the anti-inflammatory Treg and dendritic cell pools. Inhibition of inflammasome activation and dietary changes to reverse dysbiosis could open new therapeutic perspective for IBD in CGD.
Patients carrying the X-linked CYBB/GP91PHOX mutation of NOX2 develop a more severe IBD at an earlier age than those carrying the autosomal NCF1/P47PHOX mutation. The genotype shapes the inflammatory response against colitogenic microbiota and determines the severity of colitis, by altering mucus production, activating the inflammasome, and reducing the anti-inflammatory Treg and dendritic cell pools. Inhibition of inflammasome activation and dietary changes to reverse dysbiosis could open new therapeutic perspective for IBD in CGD.
Conflict-of-interest disclosure: The authors declare no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal