Issue Archive
Table of Contents
BLOOD COMMENTARIES
HOW I TREAT
How I treat dysfibrinogenemia
Casini and de Moerloose provide a comprehensive guide to the evaluation and treatment of congenital dysfibrinogenemia. With 5 illustrative cases, they delineate their approach to diagnosis, management of bleeding and thrombosis, perisurgical management, and approach to pregnancy in patients with this disorder.
CLINICAL TRIALS AND OBSERVATIONS
A phase 3, open-label, randomized study of asciminib, a STAMP inhibitor, vs bosutinib in CML after 2 or more prior TKIs
Clinical Trials & Observations
Asciminib is a novel tyrosine kinase inhibitor (TKI) targeting the myristoyl pocket of BCR-ABL rather than the ATP site, thereby potentially overcoming resistance of chronic myeloid leukemia (CML) to other TKIs. Réa et al present a phase 3 trial comparing asciminib to bosutinib in CML patients resistant to ≥2 other TKIs, reporting a major molecular response rate with asciminib of 25.5% as compared to 12.2% with bosutinib, and a favorable safety profile. These data support asciminib use for the treatment of CML resistant to prior TKI therapy.
Ponatinib dose-ranging study in chronic-phase chronic myeloid leukemia: a randomized, open-label phase 2 clinical trial
Clinical Trials & Observations
The original phase 2 PACE trial of ponatinib for chronic myeloid leukemia (CML) resistant to other tyrosine kinase inhibitors (TKI) showed efficacy but was stopped prematurely because of a high incidence of dose-dependent arterial occlusion. Cortes and colleagues report the efficacy and safety of 3 dosing strategies for ponatinib in a new trial, showing that a full starting dose of 45 mg, reducing to 15 mg after response, provides excellent efficacy and reduced toxicity.
HEMATOPOIESIS AND STEM CELLS
Dopamine signaling regulates hematopoietic stem and progenitor cell function
Hematopoietic stem and progenitor cells (HSPCs) are regulated by signals from the marrow niche. Liu et al delineate a novel signalling pathway for HSPCs by which sympathetic nerve-derived dopamine regulates HSPCs by upregulating the kinase Lck, which in turn regulates mitogen-activated protein kinase modulation of stem cell factor signaling. This critical role for dopamine potentially could be exploited therapeutically to improve HSPC expansion following stem cell transplantation or recovery from marrow-suppressive insults.
LYMPHOID NEOPLASIA
miR-130b and miR-128a are essential lineage-specific codrivers of t(4;11) MLL-AF4 acute leukemia
MLL-AF4 acute leukemia is an aggressive malignancy seen in infants and pediatric patients. Malouf et al provide new mechanistic information eludicidating disease progression. They report two microRNAs that are direct downstream targets of MLL-AF4, and demonstrate that they are sufficient to drive the development and maintenance of leukemic blasts. They further show that 2 downstream targets suppressed by the microRNAs are tumor suppressors of MLL-AF4 leukemia. These observations provide a platform for developing novel therapies for this devastating leukemia.
MYELOID NEOPLASIA
Clinical relevance of clonal hematopoiesis in persons aged ≥80 years
Clinical Trials & Observations
PLATELETS AND THROMBOPOIESIS
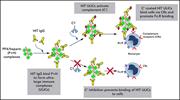
Complement mediates binding and procoagulant effects of ultralarge HIT immune complexes
Current treatment of patients with heparin-induced thrombocytopenia (HIT) with direct thrombin inhibition is effective, but some patients have an inadequate response. Khandelwal et al report that complement has a major role in HIT by physically reacting with ultralarge immune complexes composed of immunoglobulin G antibodies to platelet factor 4 and heparin, facilitating interaction of activated C3 with neutrophils and monocytes and promoting platelet activation and thrombosis. These data suggest that complement inhibition could facilitate treatment of HIT.
RED CELLS, IRON, AND ERYTHROPOIESIS
Somatic uniparental disomy mitigates the most damaging EFL1 allele combination in Shwachman-Diamond syndrome
THROMBOSIS AND HEMOSTASIS
Elevated plasma concentration of complement factor C5 is associated with risk of future venous thromboembolism
Clinical Trials & Observations
Skjeflo and colleagues studied the role of complement in the pathogenesis of venous thromboembolism (VTE). They examined whether genetic variants or chronic inflammation influence the level of complement component C5, and they investigated whether C5 levels influence VTE risk. Whole-genome sequencing and protein quantitative trait loci analysis revealed no genomic influence on C5 level. Elevated C5 levels are linked to inflammation and higher VTE risk, especially unprovoked events.
LETTERS TO BLOOD
Out-of-specification tisagenlecleucel does not compromise safety or efficacy in pediatric acute lymphoblastic leukemia
Clinical Trials & Observations
Impact of NFE2 mutations on AML transformation and overall survival in patients with myeloproliferative neoplasms
Clinical Trials & Observations
Marcault et al report the impact of NFE2 mutations on the prognosis of patients with myeloproliferative neoplasms (MPNs) in a study of over 700 patients for whom sequential next-generation sequencing was available. NFE2 mutations were reported in 9.1% of patients and predicted for increased transformation and decreased survival. Since histone deacetylase (HDAC) inhibitors decrease NFE2 levels, patients with NFE2 mutations may benefit from HDAC inhibitors.
BLOOD WORK
-
Cover Image
Cover Image
![issue cover]()
Confocal microscopic image of tissue section showing proliferation (EdU [5-ethynyl-2′-deoxyuridine]; red) of transplanted hematopoietic cells (GFP; green) and endomucin-positive endothelial cells (blue) in murine bone marrow. See the article by Liu et al on page 2051.
- PDF Icon Front MatterFront Matter
- PDF Icon Table of ContentsTable of Contents
- PDF Icon Back MatterBack Matter
- PDF Icon Editorial BoardEditorial Board
Advertisement intended for health care professionals
Email alerts
Advertisement intended for health care professionals











Asciminib for CML: same target, new arrow
Clinical Trials & Observations