In this issue of Blood, Khatib-Massalha et al show that upregulation of CD24 on JAK2V617F neutrophils inhibits their clearance, leading to an accumulation of senescent neutrophils that interact with megakaryocytes (MKs) to induce the release of active transforming growth factor β1 (TGF-β1) and the development of myelofibrosis (MF).1
MF is usually the most severe stage of BCR::ABL1-negative classic myeloproliferative neoplasms (MPNs) and can be either primary (PMF) or secondary to polycythemia vera (PV) or essential thrombocythemia (ET). MPNs are driven by 3 major gene mutations, namely in JAK2, calreticulin (CALR), and MPL, and regardless of the type of mutation, MF can develop, suggesting that myeloproliferative leukemia (MPL) signaling plays a central role in the development of bone marrow fibrosis. This hypothesis is further supported by the fact that thrombopoietin (TPO) can induce bone marrow fibrosis in humans and a lethal disease in mice that mimics PMF.2 However, the exact mechanism of bone marrow fibrosis in MPNs is still unclear, and detailed knowledge of the mechanisms involved will be important for the development of new therapeutic approaches for PMF, a disease for which there is no effective therapy. For many years, MKs have been considered the key cell type in the development of bone marrow fibrosis. More recently, the contribution of monocytes/macrophages to the generation of fibrocytes, a stromal cell involved in the production of collagen fibers, has been recognized.3
Among cytokines, TGF-β1 is essential for the development of bone marrow fibrosis as it is for fibrosis in other organs. In addition, other inflammatory profibrotic cytokines are also involved by multiple mechanisms, including the reprogramming of mesenchymal stromal cells to myofibroblasts.4
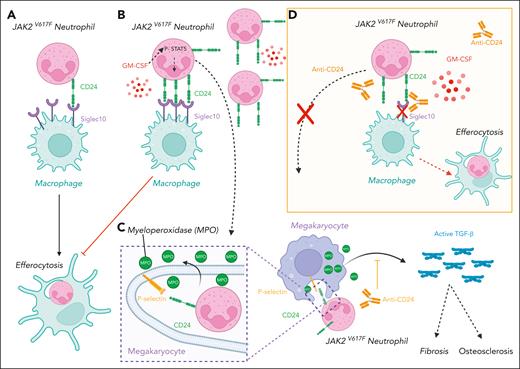
Here, the authors used mouse models and human samples coupled with sophisticated cultures in a 3-dimensional bioscaffold to show that senescent neutrophils are a new key determinant in the development of bone marrow fibrosis in JAK2V617F MPNs and unexpectedly in the mechanism of thrombocytosis. The increase of neutrophils in JAK2V617F MPNs has been mainly considered as the consequence of an increased production by constitutive activation of the granulocyte-colony-stimulating factor receptor. Here, Khatib-Massalha et al show that senescent granulocytes accumulate in JAK2V617F MPNs as a consequence of a defective efferocytosis by macrophages, which is the hemostatic mechanism of neutrophil clearance (see figure panels A-B). This accumulation is the consequence of an upregulation of CD24, which mediates a “Don’t eat me” signal, and not of CD47, the usual molecule involved in such signaling. In fact, CD24, a glycosylphosphatidylinositol-anchored glycoprotein, has recently been identified as a key molecule in the development of solid tumors, such as breast cancer, and in hematological malignancies, such as lymphoma.5 In addition to inducing multiple signaling pathways, CD24 binds to Siglec, more particularly Siglec 10, on macrophages to inhibit phagocytosis, including the elimination of tumor cells. Neutrophils normally express low levels of CD24, but in this study it was found to be upregulated in JAK2V617F neutrophils as a consequence of increased JAK2/STAT5 signaling by elevated granulocyte-macrophage colony-stimulating factor (GM-CSF) levels in the plasma of patients with JAK2V617F MPN and in a mouse model (see figure panels A-B).1 Interestingly, JAK2V617F alone or granulocyte colony-stimulating factor, which essentially activates the JAK2/STAT3 pathway, is unable to increase CD24 expression. Thus, this upregulation of neutrophil CD24 is an indirect effect of JAK2V617F via increased synthesis of GM-CSF.
Upregulation of CD24 inhibits the clearance of senescent JAK2V617F neutrophils, which interact with megakaryocytes to induce a myelofibrosis and an osteosclerosis. (A-B) JAK2V617F neutrophils express low levels of CD24 at baseline (A), but JAK2V617F increases GM-CSF levels, which, in turn, upregulates CD24 (B). (B) These increased levels of CD24 inhibit the process of efferocytosis by macrophages and thereby the clearance of senescent neutrophils that accumulate in JAK2V617F MPN. (C) These senescent neutrophils interact with megakaryocytes through the binding of CD24 on P-selectin located on demarcation membranes (emperipolesis). This interaction activates TGF-β1 through enzymes such as myeloperoxidase, which induces bone marrow fibrosis and osteosclerosis by activating myofibroblasts and osteoblasts. (D) Anti-CD24 blocking antibodies reverse the process.
Upregulation of CD24 inhibits the clearance of senescent JAK2V617F neutrophils, which interact with megakaryocytes to induce a myelofibrosis and an osteosclerosis. (A-B) JAK2V617F neutrophils express low levels of CD24 at baseline (A), but JAK2V617F increases GM-CSF levels, which, in turn, upregulates CD24 (B). (B) These increased levels of CD24 inhibit the process of efferocytosis by macrophages and thereby the clearance of senescent neutrophils that accumulate in JAK2V617F MPN. (C) These senescent neutrophils interact with megakaryocytes through the binding of CD24 on P-selectin located on demarcation membranes (emperipolesis). This interaction activates TGF-β1 through enzymes such as myeloperoxidase, which induces bone marrow fibrosis and osteosclerosis by activating myofibroblasts and osteoblasts. (D) Anti-CD24 blocking antibodies reverse the process.
This senescence profoundly modifies the cell interaction properties of neutrophils, in particular their trafficking within MKs, a process called emperipolesis. In fact, MKs have an open network of membranes called demarcation membranes in which bone marrow cells, in particular neutrophils, can traffic. It has been shown that the increased neutrophil emperipolesis mediated by P-selectin may play a central role in the development of bone marrow fibrosis not only in MPN, but also in gray platelet syndrome.6,7 In fact, emperipolesis is not a passive process because it leads to increased interaction of neutrophils with MKs, resulting in an exchange of proteins and mRNA.8 In particular, neutrophil myeloperoxidase and elastase are able to activate the latent TGF-β1 secreted by MKs in the bone marrow environment leading to the development of bone marrow fibrosis and osteosclerosis by activating myofibroblasts and osteoblasts, respectively (see figure panel C).
Khatib-Massalha et al have inhibited CD24 using a genetic approach or antibodies and showed that this reduced the number of senescent neutrophils. In addition, CD24 blockade not only reduced emperipolesis by altering the binding of neutrophil CD24 to MK P-selectin, but also prevented the development of myelofibrosis and significantly reduced osteosclerosis in JAK2V617F mice (see figure panel D). These 2 important effects correlated with a decrease of active TGF-β1 levels. Unexpectedly CD24 gene ablation also abolished thrombocytosis in JAK2V617F mice, whereas it had no effect on the platelet levels in wild-type mice. The authors were able to explain this normalization by showing that senescent neutrophils enhance proplatelet formation in vitro, thereby increasing platelet production. They suggest that this increased proplatelet formation is driven by both the direct interaction of senescent neutrophils with MKs and the increased activation of TGF-β1. Such a hypothesis is somewhat provocative because it is known that TGF-β1 generally inhibits megakaryopoiesis by directly acting on MK progenitors and MKs and by suppressing TPO production in the liver,9 although it can also increase proplatelet formation in vitro.10 Thus, it will be important to further investigate the effects of TGF-β1 on JAK2V617F megakaryopoiesis.
Finally, Khatib-Massalha et al show that the increased number of senescent neutrophils seems to be restricted to JAK2V617F MPN as it was absent in CALR-mutated MPN. Thus, these results suggest that the physiopathology of bone marrow fibrosis and of thrombocytosis differs among MPNs and depends on the driver mutation despite the common constitutive activation of the MPL/JAK2/STAT pathway.
Currently, MPNs are classified into 3 diseases, PV, ET and PMF, based on clinical phenotype. With the development of new therapies targeting the driver mutations and with the possibility that the physiopathology of myelofibrosis is driver-mutation dependent, a classification based on the type of driver mutation as in acute myeloid leukemia may be an interesting alternative.
In conclusion, this innovative work underscores the role of senescent neutrophils in the thrombocytosis and bone marrow fibrosis of JAK2V617F MPN and highlights CD24 as a new attractive therapeutic target for JAK2V617F MF.
Conflict-of-interest disclosure: The authors declare no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal