Issue Archive
Table of Contents
EDITORIAL
Introduction to a review series on molecular mechanisms of hematologic malignancies
To reach the goal of curing currently incurable hematologic malignancies, we need to go beyond focusing on single gene mutatons and gain deeper understanding of the consequences of genetic alterations on gene-regulatory pathways. Edited by John Crispino, these 5 cutting-edge reviews from leaders in the their fields not only summarize our current understanding of key pathways that contribute to myeloid malignancies, but also discuss new therapeutic avenues related to them. They provide a springboard for further groundbreaking basic and clinical advances in hematologic malignancies.
BLOOD COMMENTARIES
REVIEW SERIES
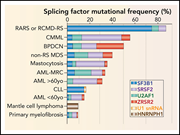
Splicing factor mutations in hematologic malignancies
To reach the goal of curing currently incurable hematologic malignancies, we need to go beyond focusing on single gene mutatons and gain deeper understanding of the consequences of genetic alterations on gene-regulatory pathways. Edited by John Crispino, these 5 cutting-edge reviews from leaders in the their fields not only summarize our current understanding of key pathways that contribute to myeloid malignancies, but also discuss new therapeutic avenues related to them. They provide a springboard for further groundbreaking basic and clinical advances in hematologic malignancies.
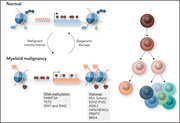
Epigenetic deregulation in myeloid malignancies
To reach the goal of curing currently incurable hematologic malignancies, we need to go beyond focusing on single gene mutatons and gain deeper understanding of the consequences of genetic alterations on gene-regulatory pathways. Edited by John Crispino, these 5 cutting-edge reviews from leaders in the their fields not only summarize our current understanding of key pathways that contribute to myeloid malignancies, but also discuss new therapeutic avenues related to them. They provide a springboard for further groundbreaking basic and clinical advances in hematologic malignancies.
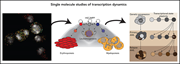
Gene expression at a single-molecule level: implications for myelodysplastic syndromes and acute myeloid leukemia
To reach the goal of curing currently incurable hematologic malignancies, we need to go beyond focusing on single gene mutatons and gain deeper understanding of the consequences of genetic alterations on gene-regulatory pathways. Edited by John Crispino, these 5 cutting-edge reviews from leaders in the their fields not only summarize our current understanding of key pathways that contribute to myeloid malignancies, but also discuss new therapeutic avenues related to them. They provide a springboard for further groundbreaking basic and clinical advances in hematologic malignancies.
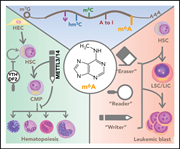
RNA modifications in hematopoietic malignancies: a new research frontier
To reach the goal of curing currently incurable hematologic malignancies, we need to go beyond focusing on single gene mutatons and gain deeper understanding of the consequences of genetic alterations on gene-regulatory pathways. Edited by John Crispino, these 5 cutting-edge reviews from leaders in the their fields not only summarize our current understanding of key pathways that contribute to myeloid malignancies, but also discuss new therapeutic avenues related to them. They provide a springboard for further groundbreaking basic and clinical advances in hematologic malignancies.
Cohesin mutations in myeloid malignancies
To reach the goal of curing currently incurable hematologic malignancies, we need to go beyond focusing on single gene mutatons and gain deeper understanding of the consequences of genetic alterations on gene-regulatory pathways. Edited by John Crispino, these 5 cutting-edge reviews from leaders in the their fields not only summarize our current understanding of key pathways that contribute to myeloid malignancies, but also discuss new therapeutic avenues related to them. They provide a springboard for further groundbreaking basic and clinical advances in hematologic malignancies.
LYMPHOID NEOPLASIA
Frequent somatic TET2 mutations in chronic NK-LGL leukemia with distinct patterns of cytopenias
Clinical Trials & Observations
Chronic natural killer large granular lymphocyte (NK-LGL) leukemia is a rare neoplasm of clonal NK cells that can be difficult to distinguish from reactive NK cell proliferations. Olson and colleagues describe genetic and epigenetic changes in 58 patients with NK-LGL leukemia, uncovering recurrent loss-of-function mutations in the TET2 gene. They highlight the associations of these lesions with altered global patterns of DNA-methylation, with distinct cytopenias, and with treatment responses.
PLATELETS AND THROMBOPOIESIS
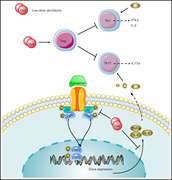
Low-dose decitabine modulates T-cell homeostasis and restores immune tolerance in immune thrombocytopenia
New treatment options are required for patients with refractory immune thrombocytopenia (ITP), a disease in which immune tolerance has been lost due to reduced regulatory T-cell (Treg) activity. Han et al demonstrate that the hypomethylating agent decitabine increases platelet counts in ITP by restoring T-cell homeostasis through modulation of Tregs, increasing their numbers and enhancing their immunosuppressive function.
RED CELLS, IRON, AND ERYTHROPOIESIS
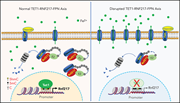
RNF217 regulates iron homeostasis through its E3 ubiquitin ligase activity by modulating ferroportin degradation
Ferroportin (FPN), the only known cellular iron exporter, plays a central role in intracellular and systemic iron homeostasis, and its levels are regulated by hepcidin. Using genetic screens and models, Jiang and colleagues identified an E3 ubiquitin ligase, RNF217, that promotes the degradation of FPN and that, if deleted, interferes with iron homeostasis in a cell type– dependent manner. Further, they identified the iron-responsive demethylase TET1 as a key upstream regulator of RNF217. These data further refine our understanding of iron homeostasis and how it can be manipulated.
TRANSFUSION MEDICINE
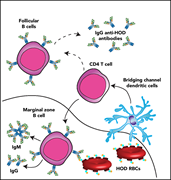
Marginal zone B cells mediate a CD4 T-cell–dependent extrafollicular antibody response following RBC transfusion in mice
Alloimmunization is a major problem in chronically transfused patients. Using transgenic red cells expressing a model surface antigen, Zerra et al uncovered a unique immune response pathway involving CD4 T cell–dependent extrafollicular immunoglobulin G response against a red cell antigen mediated by marginal zone (MZ) B cells. They also show that even transient depletion of MZ B cells can prevent alloimmunization in murine models, speaking to the potential of these discoveries to have therapeutic relevance.
TRANSPLANTATION
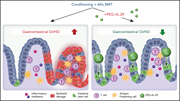
IFN-λ therapy prevents severe gastrointestinal graft-versus-host disease
Common approaches to alleviate acute graft-versus-host disease (aGvHD) focus on inducing immune tolerance by directly targeting immune cells. However, protecting key target tissues such as the gastrointestinal tract from immune-mediated damage may also reduce aGvHD severity. Using murine aGvHD models, Henden and colleagues present compelling evidence that interferon λ (IFN- λ) limits intestinal stem cell loss and promotes regeneration of gut epithelia, preserving mucosal barrier function. They propose IFN- λ therapy as a novel strategy to boost tissue protection.
BLOOD WORK
ERRATUM
-
Cover Image
Cover Image
![issue cover]()
Ferroportin (FPN; red), the unique iron exporter, can be degraded by a newly identified E3 ubiquitin ligase, RNF217. However, a truncated RNF217 (amino acids 1-384; green) is mislocated in the cell and fails to colocalize with FPN. See the article by Jiang et al on page 689.
- PDF Icon Front MatterFront Matter
- PDF Icon Table of ContentsTable of Contents
- PDF Icon Back MatterBack Matter
- PDF Icon Editorial BoardEditorial Board
Advertisement intended for health care professionals
Email alerts
Advertisement intended for health care professionals





Altered epigenetics at the center of NK-LGL leukemia