In this issue of Blood, Beri et al1 report on the impact of Babesia divergens on human red blood cells (RBCs) with hemoglobin (Hb) AA, AS, or SS. Although parasite biology is important for babesiosis, the RBC is likely to be the chief culprit of the pathophysiology of this infectious disease. The authors provide new details about how this intraerythrocytic parasite impacts RBC deformability (RBC-D), RBC sickling, and the release of extracellular vesicles (EVs) from RBCs. These new insights may explain important features of parasite infection that drive morbidity and mortality.
Babesia is an intraerythrocytic parasite that is related to the malaria-causing Plasmodium.2,3 The United States has the highest reported number of babesiosis cases of any nation. Most US cases are caused by B microti, transmitted by Ixodes scapularis ticks, and occurs most commonly in the Northeast and upper Midwest. However, this infectious disease also impacts Europe, China, and elsewhere.4 Individuals at greatest risk for severe disease include the elderly and immunosuppressed, especially those with a history of splenectomy.5 Despite the Centers for Disease Control and Prevention making babesiosis a nationally notifiable condition in 2011, understanding of this parasite and the infectious disease remains far behind that of malaria-causing Plasmodium.
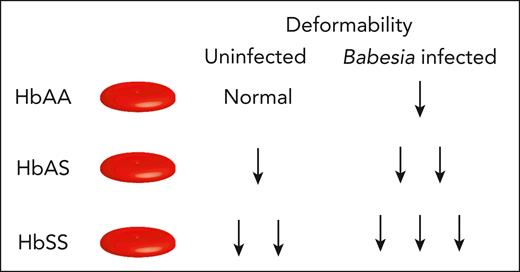
Beri et al used ektacytometry to compare the deformability of uninfected RBCs (uRBCs) to B divergens–infected RBCs (iRBCs). B divergens typically causes more severe disease than B microti, especially in immunocompromised patients, and is most common in Europe. The laser-assisted optical rotational red cell analyzer (Lorrca ektacytometer, RR Mechatronics, Zwaag, The Netherlands) can measure the elongation of RBCs in response to shear stress, osmolality, and oxygen level. The authors use this technology to show reduced RBC-D of iRBCs compared with separate samples of uRBCs in vitro, regardless of the type of Hb (see figure). iRBCs with HbSS had the lowest RBC-D, followed by HbAS and then HbAA, demonstrating that even patients with sickle cell trait may be at greater risk of complications associated with Babesiosis than individuals with HbAA.
Babesia infection impacts RBC deformability based on hemoglobin status. The degree of deformability can distinguish RBCs isolated from HbAA, HbAS, and HbSS individuals at baseline. Upon Babesia divergens infection, the deformability of RBCs from HbAA, HbAS, and HbSS further declines as indicated.
Babesia infection impacts RBC deformability based on hemoglobin status. The degree of deformability can distinguish RBCs isolated from HbAA, HbAS, and HbSS individuals at baseline. Upon Babesia divergens infection, the deformability of RBCs from HbAA, HbAS, and HbSS further declines as indicated.
The authors found that increasing parasitemia led to reduced RBC-D, regardless of Hb type. Although higher oxygenation can increase parasitemia,6 it also increases HbSS RBC-D. Higher parasitemia led to increased chance of sickling of HbSS iRBCs, as shown using sickle cell imaging flow cytometry assay. Imaging flow cytometry was also used to measure the surface area of uRBCs and iRBCs with the different hemoglobin types. iRBCs with HbSS had a greater loss of surface area compared with uRBCs with HbSS. Using tunable resistive pulse sensing, EV release was found to be greater in iRBCs than uRBCs. Interestingly, iRBCs with HbSS generated more EVs than those with HbAA, despite lower parasitemia.6 Loss of cell membrane in iRBCs with HbSS may partially contribute to their loss of RBC-D.
These results are in line with reports of reduced RBC-D of Plasmodium falciparum, P knowlesi, and P vivax iRBCs performed using ektacytometry.7,8 Reduced RBC-D can contribute to disease pathogenesis by causing microvascular obstruction and organ damage, but it also makes the iRBCs less able to navigate interendothelial slits of the spleen and recirculate. Thus, there seems to be both beneficial and harmful effects to the patient.9 However, as sickle cell disease is associated with splenic infarction and functional asplenia, it is possible that the rigid Babesia iRBCs may be more likely to persist in the circulation causing a more severe form of babesiosis. In addition, individuals with HbSS often receive RBC transfusions (simple or exchange transfusions) as a treatment or risk reduction strategy for stroke and other complications. Thus, they may be at increased risk of transfusion-transmitted Babesia infection because US Food and Drug Administration guidance only recommends Babesia testing for blood donors in the highest risk areas. Consequently, the risk has not been eliminated.
The authors’ research leads to several interesting questions. Do blood types and other RBC variables impact these outcomes like reports on Plasmodium infection? Does B microti lead to similar effects? Will RBCs isolated from patients infected with babesiosis exhibit similar characteristics to those observed in the study’s findings? What is the role of iRBC- and uRBC-derived EVs in the pathogenesis of babesiosis? Are EVs derived from iRBCs incorporated into uRBCs or taken up by immune cells or endothelial cells10? Do the EVs impact the patient’s immune response or blood flow?
One limitation of this work is that ektacytometry measures RBC-D of the entire RBC population. In contrast, micropipette approaches could be used to measure RBC-D for individual cells.7 Although this is not a trivial approach, and certainly beyond the scope of the present study, it will be of interest in future studies to determine whether uRBCs exhibit similar reductions in RBC-D in the same sample as iRBCs . Ultimately, Beri et al provide a major contribution to our understanding of babesiosis and set the stage for important future research into how Babesia infection contributes to disease pathogenesis.
Conflict-of-interest disclosure: S.R.S. has consulted for Alexion, Novartis, and Cellics; received honoraria from Grifols; and received a grant from Alexion. However, none of these are directly related to this work. R.P.J. declares no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal