In this issue of Blood, Carreño-Tarragona et al1 reveal a novel interaction between the JAK2 46/1 haplotype and PD-L1 expression, offering new insights into the molecular mechanisms underlying myeloproliferative neoplasm (MPN) susceptibility and risk of progression. Using a high-throughput technique to explore the 3-dimensional organization of the genome (circular chromosome conformation capture [4c-seq]), the authors identified a region within the JAK2 gene that transcriptionally regulates PD-L1 expression. This study provides crucial insights into why the 46/1 haplotype potentially increases the risk of MPN initiation upon acquisition of the JAK2 V617F mutation, providing key insights into preventing disease onset and managing its progression (see figure).
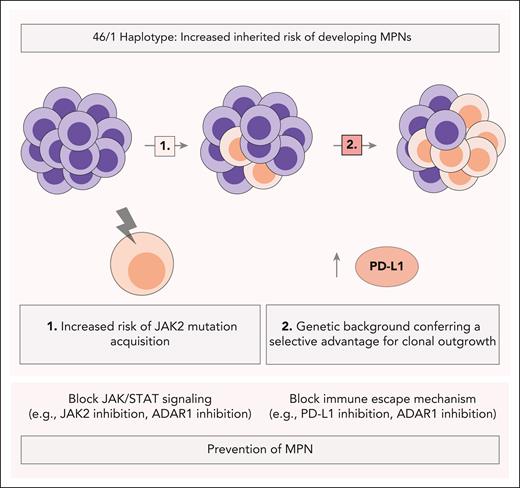
JAK2 46/1 haplotype proposed mechanisms in MPN risk and progression. The JAK2 46/1 haplotype increases the inherited risk of developing MPNs through 2 proposed mechanisms: (1) it predisposes carriers to the acquisition of JAK2 mutations, and (2) its genetic background provides a selective advantage for clonal outgrowth. In this context, MPN-derived stem cells with high PD-L1 expression may establish an immunosuppressive microenvironment in the bone marrow, promoting disease progression. These mechanistic insights may inform the development of diagnostic and therapeutic strategies aimed at preventing MPN progression, including blocking immune escape mechanisms (eg, PD-L1 inhibition, ADAR1 inhibition) alongside targeting JAK/STAT signaling (eg, JAK2 inhibition, ADAR1 inhibition).
JAK2 46/1 haplotype proposed mechanisms in MPN risk and progression. The JAK2 46/1 haplotype increases the inherited risk of developing MPNs through 2 proposed mechanisms: (1) it predisposes carriers to the acquisition of JAK2 mutations, and (2) its genetic background provides a selective advantage for clonal outgrowth. In this context, MPN-derived stem cells with high PD-L1 expression may establish an immunosuppressive microenvironment in the bone marrow, promoting disease progression. These mechanistic insights may inform the development of diagnostic and therapeutic strategies aimed at preventing MPN progression, including blocking immune escape mechanisms (eg, PD-L1 inhibition, ADAR1 inhibition) alongside targeting JAK/STAT signaling (eg, JAK2 inhibition, ADAR1 inhibition).
As clonal hematopoietic stem cell–derived disorders that often arise from JAK2 signaling-inducing mutations that can progress to secondary acute myeloid leukemia at variable rates, MPNs are influenced by macroenvironmental stressors and inflammatory cytokines.2 With a prevalence of 295,000 individuals, MPNs represent a significant cause of morbidity and mortality as well as a major health care economic burden in the United States.3 Despite major advances in JAK2 inhibitor-targeted therapeutics, the main clinical challenge pertains to predicting and preventing MPN development and progression. However, what if we could prevent MPN development in the first place?
The 46/1 haplotype has been associated with an increased risk of developing JAK2-mutated MPNs.4-6 In this study by Carreño-Tarragona et al, the authors link immune checkpoint regulation to the genetic background provided by the 46/1 haplotype, a connection not previously established in this context. Their study suggests that MPN-derived stem cells with high PD-L1 expression may create an immunosuppressive microenvironment in the bone marrow, facilitating clonal expansion of JAK2-mutated cells, which could be a key mechanism underlying the genetic predisposition to MPNs. Moreover, circular chromosome conformation capture revealed a physical interaction between PD-L1 and JAK2. This interaction differs between the 46/1 haplotype and nonrisk haplotypes, suggesting a novel chromatin conformation that might play a role in the genetic regulation of PD-L1.
Although the study by Carreño-Tarragona et al reports that PD-L1 expression is elevated in 46/1 haplotype carriers, the precise mechanistic role of PD-L1 in MPN pathogenesis remains unclear. PD-L1 is a key player in immune checkpoint regulation and immune evasion, however, its contribution to MPN initiation or progression is not fully understood. Does PD-L1 upregulation promote immune evasion specifically in 46/1-haplotype carriers or is it simply a biomarker of susceptibility? Future studies should aim to clarify whether PD-L1 directly contributes to the development of MPN in these individuals.
Additionally, Carreño-Tarragona et al suggest but do not completely elucidate a mechanism that explains the increased risk for acquisition of JAK2 V617F mutation in the 46/1 haplotype, as the PD-1/PD-L1 pathway has only been implicated in playing a role in MPN development after acquisition of JAK2 mutation.7 Two nonexclusive hypotheses could explain this: (1) the mutation forms more easily in the 46/1 haplotype or (2) it confers a selective advantage once present. Interestingly, previous findings from our laboratory and other groups have demonstrated that inflammatory cytokine-inducible APOBEC3C C-to-T DNA editing activation promotes MPN progression, suggesting that MPN-derived hematopoietic stem and progenitor cells may be more prone to genomic instability.8 This raises the question of whether APOBEC3C-mediated mutagenesis might contribute to the acquisition of JAK2 V617F in the context of the 46/1 haplotype, a potential mechanism that has yet to be investigated. Moreover, inflammatory cytokine signaling through STAT3 has been shown to activate PD-L1 transcription, thereby suggesting that additional pathways may be involved in MPN progression in inflammatory niches.
Notably, Carreño-Tarragona et al demonstrate that parallels may be drawn between the mechanisms underlying MPN development and those associated with its progression. Specifically, the current study suggests that PD-L1 plays a critical role in MPN initiation while another inflammatory cytokine-inducible editing enzyme, ADAR1, has been implicated in disease progression. Because PD-L1 expression allows chronic inflammation to persist unchecked, the cytokine-inducible ADAR1 isoform, ADARp150, is upregulated in response to chronic inflammation.7 Notably, studies have shown that ADAR1 knockdown can overcome resistance to immune checkpoint inhibitor-based immunotherapy in preclinical tumor models.9,10 Collectively, these mechanistic insights highlight new diagnostic, prognostic, and therapeutic opportunities for both the prevention of MPNs and the management of MPN progression.
Conflict-of-interest disclosure: C.H.M.J. is a cofounder of Impact Biomedicines and Aspera Biomedicines, and has received royalties from Forty Seven Inc. I.v.d.W. declares no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal