Issue Archive
Table of Contents
BLOOD COMMENTARIES
BLOOD SPOTLIGHT
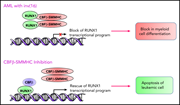
Emerging therapies for inv(16) AML
Although inv(16) AML is considered a low-risk AML, more than two-thirds of older patients relapse, especially those who cannot tolerate high-dose consolidation therapies that are critical to disease control. In a Blood Spotlight, Surapally et al highlight the current understanding of the molecular pathophysiology of the fusion oncogene CBFβ-SMMHC in driving inv(16) AML and discuss how that understanding informs potential novel therapies to improve disease prognosis.
CLINICAL TRIALS AND OBSERVATIONS
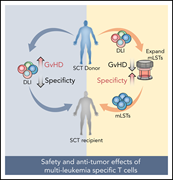
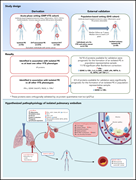
Clinical effects of administering leukemia-specific donor T cells to patients with AML/MDS after allogeneic transplant
Clinical Trials & Observations
Separating graft-versus-leukemia (GVL) from graft-versus-host disease (GVHD) has been the holy grail for preventing leukemic relapse following hematopoietic cell transplant (HCT). Lulla et al report successful expansion of donor T cells selected for leukemia-specific antigens and infusion into 25 patients with high-risk or relapsed acute myeloid leukemia (AML) following HCT. Infusions did not increase GVHD and had in vivo anti-leukemic activity. These early results raise hope for successfully separating GVL and GVHD.
GENE THERAPY
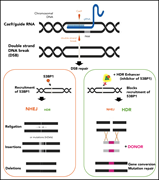
Enhanced homology-directed repair for highly efficient gene editing in hematopoietic stem/progenitor cells
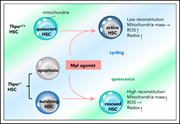
HEMATOPOIESIS AND STEM CELLS
IMMUNOBIOLOGY AND IMMUNOTHERAPY
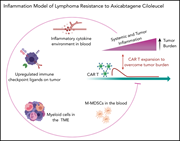
Tumor interferon signaling and suppressive myeloid cells are associated with CAR T-cell failure in large B-cell lymphoma
Over half of patients who respond to chimeric antigen receptor (CAR) T-cell therapy relapse, and although some relapses are associated with target antigen loss, there is strong evidence that immune dysregulation plays an important role in CAR T-cell failure. Jain and colleagues examined the impact of inflammatory signaling in lymphoma cells and tumor-associated macrophages and myeloid-derived suppressor cells (MDSCs) on CAR-T success. They demonstrate that high levels of interferon signaling are associated with increased macrophages, MDSCs, and increased PD-L1 expression and predict for therapy failure.
LYMPHOID NEOPLASIA
Final results of a phase 1 study of loncastuximab tesirine in relapsed/refractory B-cell non-Hodgkin lymphoma
Clinical Trials & Observations
Hamadani et al report results of a phase 1 study of loncastuximab tesirine, a CD19 antibody–drug conjugate, in the treatment of relapsed/refractory non-Hodgkin lymphoma. They establish a phase 2 dosing schedule with acceptable toxicity. They report an overall response rate of 45.6% with 26.7% complete responses, encouraging further study of this agent for treatment of relapsed lymphoma.
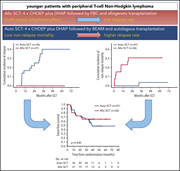
A randomized phase 3 trial of autologous vs allogeneic transplantation as part of first-line therapy in poor-risk peripheral T-NHL
Clinical Trials & Observations
Schmitz and colleagues report results of a phase 3 trial investigating whether consolidative allogeneic transplantation (alloSCT) is superior to autologous transplantation (autoSCT) for management of patients with peripheral T-cell lymphoma. No patients proceeding to alloSCT relapsed, compared with 36% of patients undergoing autoSCT. However, disease control is offset by nonrelapse mortality, with overall survival favoring autoSCT. The authors conclude that treatment of choice remains chemotherapy followed by autoSCT, with alloSCT reserved for patients relapsing after autoSCT.
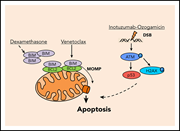
Venetoclax and dexamethasone synergize with inotuzumab ozogamicin–induced DNA damage signaling in B-lineage ALL
Brief Report
PLATELETS AND THROMBOPOIESIS
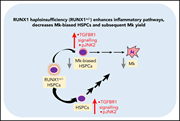
RUNX-1 haploinsufficiency causes a marked deficiency of megakaryocyte-biased hematopoietic progenitor cells
RED CELLS, IRON, AND ERYTHROPOIESIS
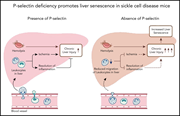
P-selectin deficiency promotes liver senescence in sickle cell disease mice
Brief Report
P-selectin inhibition prevents sickle cell disease (SCD)–associated vaso-occlusion, and the P-selectin inhibitor crizanlizumab has been approved by the US Food and Drug Administration to treat patients with SCD. Vats et al examined the impact of P-selectin knockout in SCD mice and demonstrated that although vaso-occlusion is reduced, hepatobiliary injury and fibrosis are increased. They emphasize the necessity of investigating the long-term impact of crizanlizumab on liver pathophysiology.
THROMBOSIS AND HEMOSTASIS
Protein expression profiling suggests relevance of noncanonical pathways in isolated pulmonary embolism
Clinical Trials & Observations
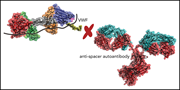
N-glycan–mediated shielding of ADAMTS13 prevents binding of pathogenic autoantibodies in immune-mediated TTP
Brief Report
Immune-mediated thrombotic thrombocytopenic purpura (iTTP) is caused by autoantibodies to ADAMTS13, most of which target the spacer domain of ADAMTS13. Using bioinformatic modeling, Ercig et al designed a series of N-glycan insertions to interfere with antibody binding and identified a variant that binds without reducing ADAMTS13 proteolytic activity. They propose this variant as a possible novel therapeutic option for iTTP treatment.
TRANSFUSION MEDICINE
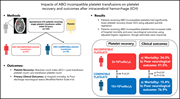
Impacts of ABO-incompatible platelet transfusions on platelet recovery and outcomes after intracerebral hemorrhage
Clinical Trials & Observations
Brief Report
Platelet transfusions are generally not type specific, and ABO-incompatible platelet transfusions are the norm. Magid-Bernstein and colleagues investigated the impact of ABO-incompatible platelet transfusion on outcomes after intracerebral hemorrhage. Despite no significant difference in hemorrhage extension, the transfusion of ABO-incompatible platelets is associated with markedly poorer platelet recovery and significantly worse neurologic recovery and survival.
LETTER TO BLOOD
Treatment dependence of prognostic gene expression signatures in de novo follicular lymphoma
Clinical Trials & Observations
BLOOD WORK
-
Cover Image
Cover Image
![issue cover]()
Quantitative liver intravital imaging of a humanized sickle cell disease (SCD) mouse injected with TXR-dextran (red) and carboxyfluorescein (green) to mark the blood flow and bile duct, respectively. Ischemic areas are evident as black voids due to the absence of vascular dye (red), suggestive of vaso-occlusion in SCD mouse liver at baseline. See the article by Vats et al on page 2676.
- PDF Icon Front MatterFront Matter
- PDF Icon Table of ContentsTable of Contents
- PDF Icon Back MatterBack Matter
- PDF Icon Editorial BoardEditorial Board
Advertisement intended for health care professionals
Email alerts
Advertisement intended for health care professionals




A step closer to GVL without GVHD
Clinical Trials & Observations