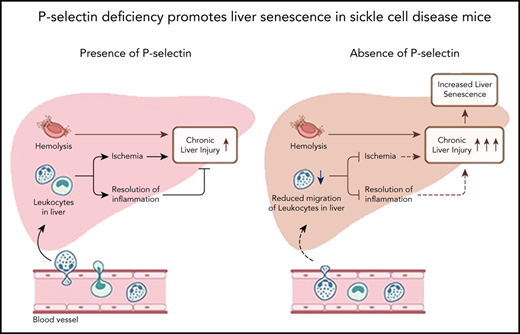
Key Points
P-selectin deficiency protects SCD mice from baseline hepatic vasoocclusion, but does not prevent chronic liver injury.
P-selectin deficiency contributes to increased cellular senescence in the liver of SCD mice.
Abstract
Sickle cell disease (SCD) is caused by a homozygous mutation in the β-globin gene, which leads to erythrocyte sickling, vasoocclusion, and intense hemolysis. P-selectin inhibition has been shown to prevent vasoocclusive events in patients with SCD; however, the chronic effect of P-selectin inhibition in SCD remains to be determined. Here, we used quantitative liver intravital microscopy in our recently generated P-selectin–deficient SCD mice to show that chronic P-selectin deficiency attenuates liver ischemia but fails to prevent hepatobiliary injury. Remarkably, we find that this failure in resolution of hepatobiliary injury in P-selectin–deficient SCD mice is associated with the increase in cellular senescence and reduced epithelial cell proliferation in the liver. These findings highlight the importance of investigating the long-term effects of chronic P-selectin inhibition therapy on liver pathophysiology in patients with SCD.
Introduction
Sickle cell disease (SCD) is caused by a homozygous mutation in the β-globin gene, which leads to erythrocyte sickling, vasoocclusion, and intense hemolysis.1 Vasoocclusion and hemolysis are the 2 predominant pathophysiologic processes in SCD that contribute to vasoocclusive pain episodes (VOE) and chronic organ damage.1-3 Previously, P-selectin inhibition/deletion was shown to attenuate acute lung vasoocclusion in transgenic humanized SCD mice administered an inflammatory stimulus.4-8 These findings were further validated by the significant reduction in VOE among SCD patients receiving the P-selectin blocking antibody.9 P-selectin antibody therapy is now US Food and Drug Administration–approved for prevention of VOE in patients with SCD; however, the chronic effect of P-selectin inhibition in SCD remains to be determined. Recently, we have shown that SCD mice manifest chronic sinusoidal ischemia and progressive liver injury under baseline conditions.10 Here, we used quantitative liver intravital microscopy (qLIM) in our recently generated P-selectin–deficient SCD mice (SS-Selp−/−)11 to show that chronic P-selectin deficiency attenuates liver ischemia but fails to prevent hepatobiliary injury. Remarkably, we found that this failure in resolution of hepatobiliary injury in SS-Selp−/−mice was associated with impaired migration of leukocytes into the liver tissue, increased cellular senescence, reduced epithelial cell proliferation, and reduced iron clearance in the liver.
Study design
Surgical preparation and intravital imaging
Details of the surgical method have been previously described.12,13 Intravascular fluorescent dyes included 200 μg of TXR-dextran, or 100 μg of carboxyfluorescein (CF). TXR-dextran was used to visualize the blood flow through the liver sinusoids, whereas CF was used to visualize the bile canaliculi. Microscopy was performed using a Nikon MPE multiphoton excitation microscope at CBI Pitt. The percentage of regions with vasoocclusion (as seen by TXR-dextran staining) per field of view (FOV) as well as number of vasoocclusion per FOVs was quantified.
Animals
Townes SS mice were bred to Selp−/− mice to generate P-selectin–deficient SS (SS-Selp−/−) mice using the breeding strategy described in Bennewitz et al11 ; Townes SCD mice (SS, homozygous for Hbatm1(HBA)Tow, homozygous for Hbbtm2(HBG1,HBB*)Tow) were used as controls. All animal experiments were approved by the Institutional Animal Care and Use Committee at the University of Pittsburgh.
Statistical analysis
All comparisons between 2 groups were deemed statistically significant by an unpaired, 2-tailed Student t test if P < .05 (*P < .05; **P < .001).
Additional methods used in this study are standard and published and are described in the supplemental Material, available on the Blood Web site.
Results and discussion
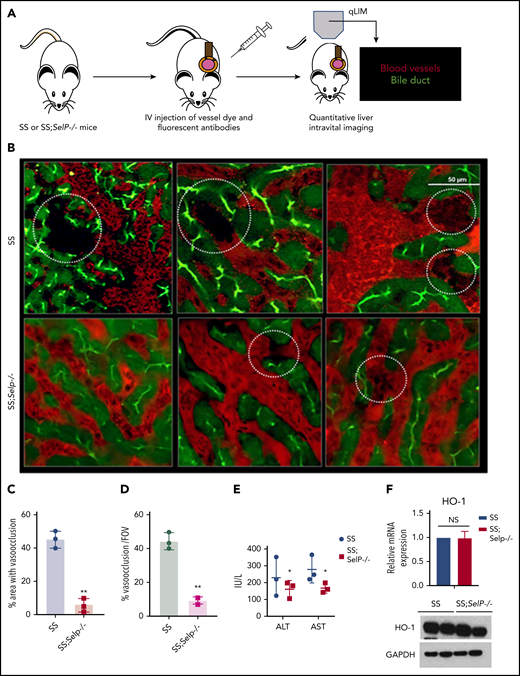
Previously, we have shown that SCD (SS) mice manifest sinusoidal ischemia and hepatobiliary injury under baseline conditions.10 Here, we have used our recently generated SS-Selp−/− mice to study the effect of chronic P-selectin inhibition on the liver pathophysiology in SCD. Identical to our previous findings, qLIM revealed sinusoidal ischemia in several regions of the liver in SS mice at baseline (Figure 1B upper panel; supplemental Videos 1-3). As shown in Figure 1B, these ischemic areas were evident as black voids in qLIM images because of the absence of vascular dye (red), suggestive of blood flow stasis. In contrast, the blood flow (red) was significantly improved within the sinusoids of SS-Selp−/− mice (Figure 1B lower panel; supplemental Videos 4-6). Remarkably, sinusoidal ischemia was significantly ameliorated in the liver of SS-Selp−/− mice under baseline conditions with ∼40% to 50% of the liver sinusoids in SS compared with only ∼5% to 10% in SS-Selp−/− mice manifesting ischemia (Figure 1C-D). Reduction in hepatic ischemia also led to mild amelioration of serum alanine aminotransferase and aspartate aminotransferase level in SS-Selp−/− mice (Figure 1E). However, similar to our previous finding,11 we found no significant change in hemolysis between SS and SS-Selp−/− mice (supplemental Table 1), which was further supported by the comparable levels of hemolysis-responsive gene heme oxygenase 1 (HO-1) in the livers of SS and SS-Selp−/− mice (Figure 1F).
P-selectin–deficient SCD mice exhibit amelioration of ischemic liver injury at baseline. (A) Schematic diagram of qLIM imaging of mice using TXR-dextran and CF to visualize blood flow and bile canaliculi, respectively. (B) qLIM images of 3 different FOVs of SS and P-selectin–deficient SCD liver (SS;Selp−/−) injected with TXR-dextran and CF. Dotted circle shows loss of blood flow in SCD liver, which was significantly improved in SS;Selp−/− liver. Original magnification, ×10; insets, ×20. (C) Quantification of the total area (%) of liver with loss of blood flow in SS and SS;Selp−/− liver. (D) Percentage of total number of vasoocclusion/FOV. (E) Serum alanine aminotransferase (ALT) and aspartate aminotransferase (AST) levels in SS and SS-Selp−/− mice. (F) Quantitative reverse transcription polymerase chain reaction (qRT-PCR) and western blot analysis show comparable amount of HO-1 in SS and SS;Selp−/− liver. *P < .05; **P < .01. GAPDH, glyceraldehyde-3-phosphate dehydrogenase; NS, not significant.
P-selectin–deficient SCD mice exhibit amelioration of ischemic liver injury at baseline. (A) Schematic diagram of qLIM imaging of mice using TXR-dextran and CF to visualize blood flow and bile canaliculi, respectively. (B) qLIM images of 3 different FOVs of SS and P-selectin–deficient SCD liver (SS;Selp−/−) injected with TXR-dextran and CF. Dotted circle shows loss of blood flow in SCD liver, which was significantly improved in SS;Selp−/− liver. Original magnification, ×10; insets, ×20. (C) Quantification of the total area (%) of liver with loss of blood flow in SS and SS;Selp−/− liver. (D) Percentage of total number of vasoocclusion/FOV. (E) Serum alanine aminotransferase (ALT) and aspartate aminotransferase (AST) levels in SS and SS-Selp−/− mice. (F) Quantitative reverse transcription polymerase chain reaction (qRT-PCR) and western blot analysis show comparable amount of HO-1 in SS and SS;Selp−/− liver. *P < .05; **P < .01. GAPDH, glyceraldehyde-3-phosphate dehydrogenase; NS, not significant.
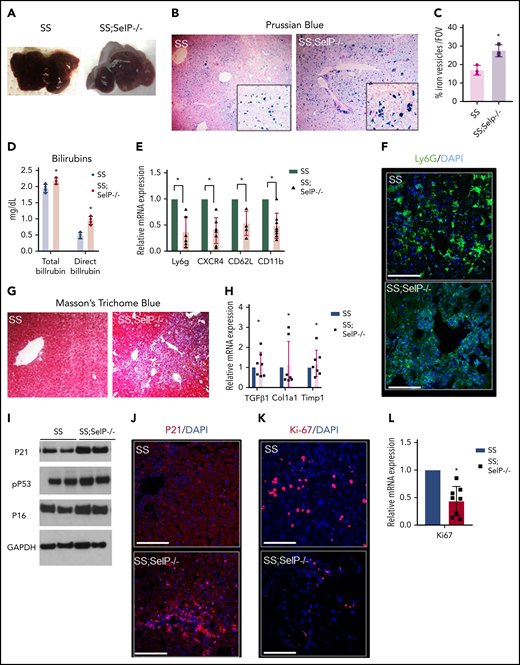
Next, we assessed the effect of P-selectin deficiency on liver injury in SS-Selp−/− mice. Previously, we have shown that liver is significantly injured in SCD mice at baseline.10 Unlike the effect on hepatic ischemia (Figure 1B), P-selectin deficiency did not prevent progressive liver injury in SCD mice. Both SS and SS-Selp−/− mice had dark red–colored livers with white spots suggestive of progressive injury (Figure 2A). Previously, we have shown the liver injury in SS mice to be associated with iron accumulation in the liver.10 Surprisingly, Prussian blue staining revealed the presence of significantly larger iron-positive vesicles in SS-Selp−/− than SS livers (Figure 2B-C), suggestive of impaired iron clearance as a result of chronic P-selectin deficiency, which was further supported by the significantly higher direct and total bilirubin in SS-Selp−/− than SS mice (Figure 2D). Interestingly, we did not see any significant change in hepcidin expression in SS-Selp−/− mice as compared with SS mice (supplemental Figure 1C-D). Iron clearance in the liver is linked to infiltration of liver inflammatory cells that promote iron trafficking in and out of the liver.14,15 Recent evidence also supports a role for monocyte/macrophage recruitment in promoting liver homeostasis and preventing ischemic liver injury in SCD mice.16,17 Therefore, we hypothesized that the large iron deposits in SS-Selp−/− mice are the result of dysregulated iron clearance, secondary to impaired recruitment of myeloid cells in absence of P-selectin. Interestingly, we11 have previously reported that the circulating myeloid cells (neutrophils and monocytes) are significantly elevated in SS-Selp−/− mice,11,18 most likely because of impaired recruitment to tissues and bone marrow. Indeed, we found significant reduction in messenger RNA (mRNA) and expression of myeloid cell markers (Ly6G, CXCR4, CD62L, and CD11b) in the liver of SS-Selp−/− than SS mice (Figure 2E), which was further confirmed by the reduction in immunofluorescence staining for neutrophil marker (Ly6g) in SS-Selp−/− compared with SS liver (Figure 2F). Inhibition of inflammatory cell recruitment in the liver may result in impaired injury resolution19-21 by delaying clearance of dead cells.22 To our surprise, immunohistochemical analysis by trichome staining (Figure 2G) showed more severe injury in the liver of SS-Selp−/− compared with SS mice, which was further confirmed by the upregulation of lactate dehydrogenase protein levels (supplemental Figure 1A-B) and mRNA encoding for TGFβ1, procollagen α1 (I), and TIMP-1 in SS-Selp−/− compared with SS mice liver (Figure 2H). Remarkably, we also found a significant increase in the protein expression of DNA damage and cell senescence markers P21, P16, and phospho-P53 (Figure 2I-J), and reduction in the hepatic proliferation marker Ki-67 (Figure 2K-L) in the liver of SS-Selp−/− mice compared with SS mice, suggesting that P-selectin deficiency in SS-Selp−/− mice impairs resolution of chronic liver injury by promoting cellular senescence and inhibiting hepatocyte proliferation upon injury. Finally, comparison of liver pathology between 3- and 8-month-old SS-Selp−/− mice suggested comparable ischemia (supplemental Figure 1E; supplemental Videos 7 and 8), senescence (supplemental Figure 1I), or proliferation (supplemental Figure 1H), but an increase in iron accumulation (supplemental Figure 1F) and liver injury (supplemental Figure 1G) with age. Although liver fibrosis was evident in SS-Selp−/− mice (Figure 2G), the possibility of progression to cirrhosis needs to be investigated in future studies. Taken together, our past and current findings suggest that chronic P-selectin deficiency in SCD mice ameliorates baseline liver sinusoidal ischemia and impairs hepatic recruitment of myeloid cells without affecting hemolysis. The reduction in myeloid cell recruitment contributes to impaired clearance of iron in the liver, leading to augmented injury and cellular senescence in the liver. Importantly, senescence is increasingly associated with cellular aging; therefore, chronic P-selectin inhibition may impair liver regeneration following hepatic crisis in SCD patients. Although previous studies17 and our current findings suggest that impaired monocyte/macrophage recruitment probably contributes to impaired iron trafficking and liver injury in SS-Selp−/− mice, the reduction in Ly6g staining (Figure 2F) suggests that P-selectin deficiency also impairs the neutrophil recruitment in the liver. Emerging evidence suggests a role for neutrophil recruitment in promoting liver homeostasis,15 thus highlighting the need for future studies aimed at elucidating how neutrophils may promote resolution of liver injury in SCD. In addition, aged proinflammatory neutrophils have been shown to contribute to vasoocclusive tissue injury in SCD mice.23 Therefore, future studies should also investigate the effect of P-selectin inhibition on the circulating aged neutrophil subpopulation and how these aged neutrophils contribute to liver senescence in SS-Selp−/− mice. A major limitation of our study is the use of SCD mice genetically deficient in P-selectin from birth to model the effects of chronic P-selectin inhibition therapy in SCD. In our current study, the elevated leukocyte counts in SS-Selp−/− mice could also be a contribution of reduced leukocyte migration into tissue or chronic (in SS-Selp−/− mice) rather than short-term (in clinical trial) P-selectin inhibition. Although the phase 2 study9 did not report any effect of crizanlizumab on circulating leukocyte counts in SCD patients, a recent case study reported a twofold increase in circulating leukocytes in an SCD patient following treatment with crizanlizumab.24 Also, the P-selectin antibody therapy in patients with SCD may not result in same extent of liver pathophysiology as caused by the genetic absence of P-selectin in SS-Selp−/− mice. Notwithstanding these limitations, our current findings highlight the need to investigate the long-term effects of chronic P-selectin inhibition therapy on circulating leukocyte populations and liver pathophysiology in patients with SCD.
SS-Selp−/− mice exhibit chronic organ injury and exacerbated senescence. (A) Gross specimen of livers of SS and SS-Selp−/− mice. Both the SS and the SS-Selp−/− mice livers were dark and manifested white spots suggestive of progressive injury. (B) Prussian blue staining for iron showed increased iron deposition with a mixed distribution in hepatocytes and Kupffer cells in SS-Selp−/− liver as compared with SS liver. (C) Quantification of iron staining/FOV. (D) Serum direct and total bilirubin levels in SS and SS-Selp−/− mice. (E) Quantitative reverse transcription polymerase chain reaction (qRT-PCR) analysis exhibit reduced mRNA expression of markers of inflammatory cells (including CXCR4, Ly6G, CD11b, and Cd62L) in SS-Selp−/− liver as compared with SS liver. (F) Immunofluorescence for Ly6G showed an increased accumulation in SS mouse liver, which was reduced in SS-Selp−/− liver. Scale bars, 20 µM. (G) Trichome staining of SS and SS-Selp−/− liver sections revealed increased perisinusoidal and periductular fibrosis in SS-Selp−/− liver. Original magnification, ×10. (H) Analysis of mRNA expression by qRT-PCR showed an increase in mRNA expression of TGFβ, Col1A1, and TIMP1 in SS-Selp−/− liver as compared with SS liver. (I) Western blot for P21, P16INK4a, and phosphor-P53 antibodies exhibits increased expression in the liver of SS-Selp−/− as compared with SS. (J) IF of P21 exhibits significant enrichment in SS-Selp−/− liver as compared with SS liver. DAPI, 4′,6-diamidino-2-phenylindole. (K) IF of Ki-67 exhibits reduced hepatocyte proliferation in SS-Selp−/− liver as compared with SS liver. Scale bars, 20 μm (J-K). (L) qRT-PCR analysis of SS and SS-Selp−/− liver exhibits reduced expression of Ki-67 in SS-Selp−/− liver as compared with SS. *P < .05.
SS-Selp−/− mice exhibit chronic organ injury and exacerbated senescence. (A) Gross specimen of livers of SS and SS-Selp−/− mice. Both the SS and the SS-Selp−/− mice livers were dark and manifested white spots suggestive of progressive injury. (B) Prussian blue staining for iron showed increased iron deposition with a mixed distribution in hepatocytes and Kupffer cells in SS-Selp−/− liver as compared with SS liver. (C) Quantification of iron staining/FOV. (D) Serum direct and total bilirubin levels in SS and SS-Selp−/− mice. (E) Quantitative reverse transcription polymerase chain reaction (qRT-PCR) analysis exhibit reduced mRNA expression of markers of inflammatory cells (including CXCR4, Ly6G, CD11b, and Cd62L) in SS-Selp−/− liver as compared with SS liver. (F) Immunofluorescence for Ly6G showed an increased accumulation in SS mouse liver, which was reduced in SS-Selp−/− liver. Scale bars, 20 µM. (G) Trichome staining of SS and SS-Selp−/− liver sections revealed increased perisinusoidal and periductular fibrosis in SS-Selp−/− liver. Original magnification, ×10. (H) Analysis of mRNA expression by qRT-PCR showed an increase in mRNA expression of TGFβ, Col1A1, and TIMP1 in SS-Selp−/− liver as compared with SS liver. (I) Western blot for P21, P16INK4a, and phosphor-P53 antibodies exhibits increased expression in the liver of SS-Selp−/− as compared with SS. (J) IF of P21 exhibits significant enrichment in SS-Selp−/− liver as compared with SS liver. DAPI, 4′,6-diamidino-2-phenylindole. (K) IF of Ki-67 exhibits reduced hepatocyte proliferation in SS-Selp−/− liver as compared with SS liver. Scale bars, 20 μm (J-K). (L) qRT-PCR analysis of SS and SS-Selp−/− liver exhibits reduced expression of Ki-67 in SS-Selp−/− liver as compared with SS. *P < .05.
For original data, please contact the corresponding author.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Mark T. Gladwin and Gregory J. Kato for helpful comments on the manuscript.
This work was supported, in part, by the National Institutes of Health (NIH)/National Institute of Diabetes and Digestive and Kidney Diseases grant 1K01DK125617-01 (T.P.-S.) and NIH/National Heart, Lung, and Blood Institute grants 1R01HL128297 (P.S.) and 1R01HL141080 (P.S.). This work was also supported, in part, by funds from the Hemophilia Center of Western Pennsylvania (P.S., T.P.-S.) and Vitalant (P.S.), and by American Heart Association award 19PRE34430188 (R.V.).
Authorship
Contribution: R.V., E.-M.J., E.T., J.T., T.W.K., T.B., and T.P.-S. conducted the experiments; R.V., E.M.-J., and T.P.-S. performed the data analysis; and T.P.-S. wrote the manuscript with suggestions, consultation, and contributions from E.M.N. and P.S.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Tirthadipa Pradhan-Sundd, Division of Hematology-Oncology, Department of Medicine, University of Pittsburgh School of Medicine, E1225 BST, 200 Lothrop St, Pittsburgh, PA 15261; e-mail: tip9@pitt.edu; and Prithu Sundd, Division of Pulmonary Allergy and Critical Care Medicine, Department of Medicine, Vascular Medicine Institute, University of Pittsburgh School of Medicine, E1225 BST, 200 Lothrop St, Pittsburgh, PA 15261; e-mail: prs51@pitt.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal