Issue Archive
Table of Contents
BLOOD COMMENTARIES
HOW I TREAT
How I treat the co-occurrence of venous and arterial thromboembolism: anticoagulation, antiplatelet therapy, or both?
Through a structured analysis of 4 illustrative cases, May and Moll describe their approach to treatment of patients with co-occurrence of arterial and venous thrombotic events. Through an analysis of the indications for antithrombotic and/or antiplatelet therapy, assessment of bleeding risk, and consideration of patient-specific factors, the authors present a cogent and informative approach to this vexing problem.
CLINICAL TRIALS AND OBSERVATIONS
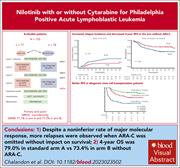
Nilotinib with or without cytarabine for Philadelphia-positive acute lymphoblastic leukemia
Clinical Trials & Observations
The introduction of tyrosine kinase inhibitors has transformed the prognosis of Philadelphia-positive acute lymphoblastic leukemia (Ph+ ALL). Chalandon and colleagues previously demonstrated that reduced-intensity chemotherapy plus imatinib was effective in induction of patients with Ph+ ALL. In the current study, the authors examined whether high-dose cytarabine (Ara-C) consolidation can be omitted from therapy. Nilotinib replaced imatinib, and 85% of patients underwent stem cell transplant and completed 2 years of nilotinib maintenance. Although major molecular response at the end of consolidation was equivalent, relapse was increased by omitting Ara-C, but overall survival was not affected.
GENE THERAPY
Vector integration and fate in the hemophilia dog liver multiple years after AAV-FVIII gene transfer
Batty et al assessed the persistence of adeno-associated virus factor VIII–(AAV-FVIII) gene transfer and the potential genotoxicity in hemophilia dogs more than 10 years after portal vein delivery of a vector. Vectors are predominantly episomal and are the source of persistent transgene expression. Rare random integrations of fragments, but not full-length vectors, occur primarily in sites of open chromatin. Insertions are not associated with gene expression changes or pathologic evidence of tumors, supporting long-term safety of the therapy.
IMMUNOBIOLOGY AND IMMUNOTHERAPY
Differential effects of itacitinib, fedratinib, and ruxolitinib in mouse models of hemophagocytic lymphohistiocytosis
The JAK1/2 inhibitor ruxolitinib has demonstrated efficacy in preclinical models and early clinical studies in patients with hemophagocytic lymphohistiocytosis (HLH), but its use raises concerns since it can also worsen cytopenias. Keenan and colleagues compared the effects of ruxolitinib to those of a selective JAK1 inhibitor (itacitinib) and a selective JAK2 inhibitor (fedratinib) in mouse models of primary and secondary HLH. Although all 3 suppressed interferon gamma-induced STAT1 phosphorylation, only ruxolitinib had an excellent therapeutic impact in both models. Neither selective inhibitor performed well in primary HLH, but itacitinib was also effective in secondary HLH.
LYMPHOID NEOPLASIA
ctDNA improves prognostic prediction for patients with relapsed/refractory MM receiving ixazomib, lenalidomide, and dexamethasone
Kogure et al report on the prognostic value of circulating tumor DNA (ctDNA) in relapsed/refractory multiple myeloma (RRMM). The authors compared targeted sequencing of bone marrow plasma cells (BMPC) to ctDNA detection in 261 uniformly treated patients with RRMM in a prospective observational study. Driver mutations associated with inferior survival were better detected by ctDNA than by targeted sequencing of BMPC, suggesting that ctDNA should become the standard for prognostic prediction in RRMM.
MYELOID NEOPLASIA
In vivo ablation of NF-κB cascade effectors alleviates disease burden in myeloproliferative neoplasms
Hyperactivation of the NF-κB cascade promotes oncogenic signaling and inflammation in the setting of myeloproliferative neoplasms (MPNs). Laranjeira and colleagues ablated factors in the NF-κB pathway in primary MPN samples and syngeneic and patient-derived xenograft mouse models to identify potential therapeutic entry points for MPN therapy. The authors identified upstream and downstream effectors that can be inhibited to suppress MPN proliferation.
THROMBOSIS AND HEMOSTASIS
Thrombosis risk in single- and double-heterozygous carriers of factor V Leiden and prothrombin G20210A in FinnGen and the UK Biobank
Clinical Trials & Observations
Ryu and colleagues investigated the thrombotic risk of double heterozygosity (DH) for factor V Leiden (FVL) and prothrombin G20210A polymorphisms, in which previous studies have disagreed. The authors used data on almost 1 million individuals in the UK Biobank and FinnGen biorepositories to evaluate venous thromboembolism (VTE) risk. They confirmed an additive risk of VTE in DH individuals, with an odds ratio of 5.2, equivalent to that for FVL homozygosity. By contrast, there was no increase in arterial thrombotic events, which is consistent with previous studies.
LETTER TO BLOOD
Endothelial ZIP8 plays a minor role in BMP6 regulation by iron in mice
The master regulator of iron homeostasis, hepcidin, downregulates iron transport in high iron states and in inflammation. Hepcidin expression by the liver is mediated by the bone morphogenetic protein (BMP)–SMAD pathway. BMP6 is induced by iron, but it is not clear how liver endothelial cells acquire iron to trigger hepcidin-inducing pathways. Fisher et al present data supporting a role for ZRT/IRT-like protein 8 (ZIP8) in iron homeostasis in the setting of elevated iron, suggesting that ZIP8 mediates the uptake of non-transferrin-bound iron.
BLOOD WORK
-
Cover Image
Cover Image
![issue cover]()
Hematoxylin and eosin–stained bone marrow in the retroviral MPLW515L mouse model of myelofibrosis. Marked osteosclerosis can be observed which, along with pathologic hallmarks, are diminished upon perturbation of NF-κB effectors including Rela and Myd88. See the article by Laranjeira et al on page 2414.
- PDF Icon Front MatterFront Matter
- PDF Icon Table of ContentsTable of Contents
- PDF Icon Editorial BoardEditorial Board
Advertisement intended for health care professionals
Email alerts
Advertisement intended for health care professionals