In this issue of Blood, Ryu and colleagues1 provide definitive data on the risk of venous thromboembolism (VTE) in individuals who are double heterozygous (DH) for factor V Leiden (FVL) and prothrombin gene mutation G20210A (PTGM). Although FVL and PTGM are the 2 most studied genetic risk factors for VTE, the risk of VTE for those with the DH genotype is less clear.
In 2001, a pooled analysis of case-control studies reported that the odds ratio (OR) for VTE in those with DH genotypes were 20.0 (95% confidence interval [CI]: 11.1-36.1), a significantly higher risk compared with heterozygous FVL (OR 4.9, 95% CI: 4.1-5.9) and heterozygous PTGM (OR 3.8, 95% CI: 3.0-4.9) individuals.2 However, an updated meta-analysis by Simone et al in 2013 found a similar risk of VTE for DH genotypes (OR 3.42, 95% CI: 1.64-7.13) as for individuals with heterozygous FVL (OR 4.22; 95% CI: 3.35-5.32) and individuals with heterozygous PTGM (OR 2.79; 95% CI: 2.25-3.46).3 This huge variance in effect size estimates for those with DH genotype is likely due to the heterogeneity of studies included in both analyses and the presence of ascertainment bias, where individuals with a prior thrombotic event or their family members are more likely to undergo thrombophilia testing.
The conflicting risk estimates present hematologists with a challenging dilemma when called upon to assess and counsel VTE risk in asymptomatic individuals with DH genotype in various clinical scenarios. These scenarios include the need for pharmacological thromboprophylaxis in individuals during pregnancy and the postpartum period or with the use of hormonal contraceptives. Despite the variable risk estimates, clinicians have erred on the side of managing individuals with DH genotype the same as those who are FVL homozygous.
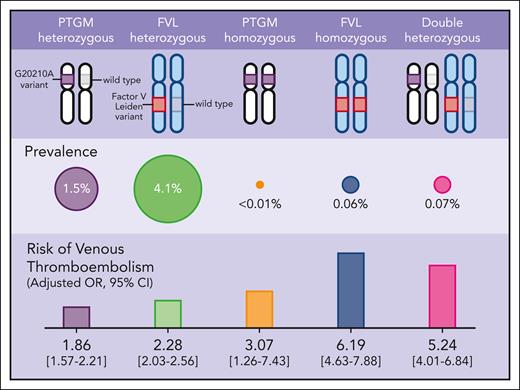
The current study by Ryu et al sheds light on this clinical dilemma. The authors leveraged 2 large population-scale genomic datasets (the UK Biobank4 and FinnGen5) comprising nearly one million participants to determine the genotypic prevalence and the risk for VTE and arterial thrombosis across FVL, PTGM, and DH genotypes (see figure). Using data from both biorepositories, the prevalence of heterozygous FVL, heterozygous PTGM, and DH genotypes were 4.1% (n = 38 186), 1.5% (n = 14 150), and 0.07% (n = 662), respectively. As for the homozygous FVL and homozygous PTGM, the prevalence was 0.06% (n = 593) and <0.01% (n = 69), respectively. After adjusting for sex, age, and ancestry, individuals with DH genotype had a markedly increased risk of VTE (OR 5.24, 95% CI: 4.01-6.84) compared with individuals with wild-type genotype, a risk that is similar to those with homozygous FVL (OR 6.19, 95% CI: 4.63-7.88). For comparison, the VTE risks associated with FVL and PTGM single heterozygosity were approximately two- to threefold higher compared with individuals with wild-type genotype and consistent with other studies.6,7 From a clinical perspective, these data support our current management of individuals with DH genotypes that parallels those who are FVL homozygous.
Genotypic prevalence and the risk for VTE across FVL, PTGM, and DH genotypes.
Genotypic prevalence and the risk for VTE across FVL, PTGM, and DH genotypes.
A notable finding of this study was that in individuals with DH genotype, the increased VTE risk from FVL and PTGM alleles behaved in an additive, rather than a multiplicative (synergistic) manner. The additive nature of the VTE risk observed suggests that other combinations of concomitant prothrombotic variants may similarly augment the overall VTE risk and does not rule out the possibility that others will behave in a synergistic manner. This underscores the importance of expanding the genomic landscape that uncovers novel variants associated with VTE, including different pathogenic variants within the same genes, as well as variants in other genes associated with VTE. Understanding the impact of different combinations of genetic variants on VTE risk and their distinct interactions could provide valuable insights. Advancements in machine learning may eventually enable the development of individualized VTE risk profiles that consider both the genetic variants and environmental risk factors for VTE to guide clinical decision-making, genetic counseling, and risk stratification tailored to the individual.
With the breadth of clinical and genomic data available from these 2 large population-scale datasets, the authors also addressed another key clinical question regarding the use of thrombophilia in the workup for arterial thrombosis. International guidelines do not recommend testing for inherited thrombophilia in individuals with arterial thrombosis. Yet, this remains common practice at many institutions, especially for stroke workup.8 The current study found no association between any of the 3 genotypes (FVL, PTGM, and DH) and myocardial infarction or stroke. This finding should further inform clinical practice that indiscriminate testing for FVL and PTGM in individuals with arterial thrombosis, particularly stroke, should be discontinued.
For future directions, a key area of investigation would be the implications of these findings across diverse populations. For instance, what is the attributable VTE risk of FVL or PTGM genotypes across different racial or ethnic groups? Such studies are currently lacking. Investigating racial disparities and prothrombotic genetic factors across diverse populations would offer valuable insights for personalized risk assessment and preventive strategies tailored to different racial or ethnic groups. This research could be pivotal not only in enhancing thrombosis prevention, but also in addressing broader health disparities. The All of Us Research Program,9 a National Institutes of Health initiative to build one of the most diverse health databases that reflects the diversity of the United States, is ongoing and may help address these questions.
Conflict-of-interest: M.Y.L. has received honoraria for participation in advisory boards with Takeda, Alexion, and Sanofi. M.Y.A.-I. has participated in advisory boards with Sanofi.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal