In this issue of Blood, Laranjeira et al1 elucidate the role of NF-ĸB signaling in myeloproliferative neoplasm (MPN) pathogenesis by demonstrating how systematic disruption of key pathway components attenuate disease burden (see figure).
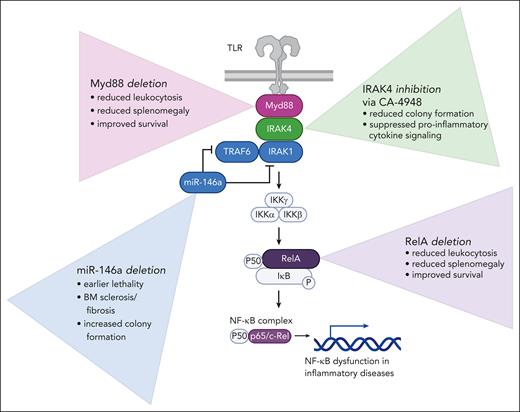
Studies performed to dissect the role of NF-ĸB in MPN pathogenesis. Affects of loss of MyD88 and RelA were investigated in the context of Jak2V617F knockin and MPLW515L transduction transplantation mouse models. Disruption of miR-146a was investigated in primary patient samples and patient-derived xenografts. The role of IRAK4 in MPN was investigated with primary patient samples, patient-derived xenografts, and mass cytometry. BM, bone marrow; TLR, Toll-like receptor.
Studies performed to dissect the role of NF-ĸB in MPN pathogenesis. Affects of loss of MyD88 and RelA were investigated in the context of Jak2V617F knockin and MPLW515L transduction transplantation mouse models. Disruption of miR-146a was investigated in primary patient samples and patient-derived xenografts. The role of IRAK4 in MPN was investigated with primary patient samples, patient-derived xenografts, and mass cytometry. BM, bone marrow; TLR, Toll-like receptor.
MPNs are myeloid malignancies driven by constitutively active JAK-STAT signaling and chronic inflammation. JAK inhibitors reduce inflammatory cytokines but not to the level of normal,2 nor do they eliminate the underlying malignant clone. This persistence suggests that other pathways contribute to clonal survival. NF-ĸB is a family of inducible transcription factors that respond to diverse stimuli. Deranged NF-ĸB function is implicated in many inflammatory diseases, including rheumatoid arthritis, inflammatory bowel disease, and atherosclerosis.3
Hyperactivation of NF-ĸB signaling is a characteristic feature of MPN, raising the question of how this intricate inflammatory pathway affects MPN pathogenesis. The NF-ĸB cascade is governed by numerous factors not yet fully evaluated in the context of MPN. The authors address this knowledge gap by using 2 syngeneic mouse MPN models, patient-derived xenografts (PDXs), and primary cells to investigate the dependency of NF-ĸB on MPN pathogenesis. With a series of comprehensive experiments systematically ablating NF-ĸB signaling pathway components, they provide novel evidence for the importance of Myd88 (myeloid differentiation factor 88) and miR-146a in MPN pathogenesis, as well as use RNA sequencing and mass cytometry to validate IRAK4 as a promising target for MPN.
First, to investigate the effect of Rela deletion on a polycythemia vera phenotype, they cross a ubiquitin C (UBC)-Cre-ERT2 mouse with 2 conditional models: Jak2V617F knockin4 and Relaflox/flox-UBC-Cre-ERT2 (Relafl/fl).5 This allows for the coincident expression of JAK2V617F along with the deletion of Rela following tamoxifen exposure. The Jak2-Relfl/fl transplanted mice cells have lower white blood cell counts, hematocrits, and spleen weights than mice transplanted with Jak2-UBC control cells. Next, the authors used an MPLW515L transduction-transplantation model to explore how loss of RelA impacts a more aggressive MPN phenotype. Hematopoietic progenitor cells (c-kit+) from Relafl/fl or UBC-control mice were transduced with MPLW515L-green fluorescent protein retrovirus, transplanted into lethally irradiated mice, and exposed to tamoxifen to induce loss of Rela. Rela ablation attenuates many of the MPLW515L-driven disease hallmarks including leukocytosis, splenomegaly, and dysmegakaryopoiesis and extends life span in transplanted mice. PDXs further define the role of RelA in humanized MPN mouse models. Purified CD34+ cells from patients with myelofibrosis (MF) and normal bone marrow donors were transduced with a single guide RNA targeting RelA. RelA loss led to a reduction in neoplastic cells in peripheral blood and bone marrow, reduced spleen weights, and improved survival in mice transplanted with MF CD34+ cells. These results show clearly and concisely that Rela is a key mediator of pathogenesis in both mild and aggressive in vivo MPN settings.
The authors also asked how Myd88 and the microRNA miR-146a affect disease severity. Myd88, often studied in infectious models, is essential in interleukin-1 receptor (IL-1R) and Toll-like receptor signaling and has been shown to promote liver fibrosis and activate hematopoietic stem cells (HSCs) and the NLRP3 inflammasome in mice.6 Hematopoietic progenitors from Myd88-null or wild-type mice were transduced with MPLW515L and transplanted into recipient mice. The Myd88-null-MPLW515L mice had attenuated leukocytosis and splenomegaly, extended survival, and reduced fibrosis as compared with the MPLW515L mice.
In contrast to the activators RelA and Myd88, the microRNA miR-146a is a repressor of NF-kB, acting as a sort of suppressive “brake.” Accordingly, ablation of miR-146a leads to excess myeloproliferation.7 Here, Laranjeira et al demonstrate that disruption of miR146a enhances colony formation of both MPN and normal controls in vitro. In PDXs, disruption of miR146a reduces survival of mice injected with MPN CD34+ cells but does not impact survival of mice injected with normal control CD34+ cells.
To bring the experimentation to a more clinically relevant setting, they perform experiments with the specific IRAK4 inhibitor, CA-4948 (emavusertib), which is currently being investigated clinically in acute myeloid leukemia (AML). The authors support their reasoning for CA-4948’s use in MPN with RNA sequencing demonstrating that MPN samples, most significantly those of MF and secondary AML, have upregulated IRAK4 compared with normal. They further validate IRAK4 as a legitimate target in MPN, evidenced by dose-dependent inhibition of colony formation in MPN samples treated with CA-4948.
This work provides a new perspective on the dependency of MPN pathogenesis on multiple NF-ĸB targets and sets the groundwork for many follow-up questions. NF-ĸB is critical for HSCs, and a 2013 study by Stein and Baldwin found that Rela-deficient HSCs performed inferiorly to wild-type HSCs.5 Here, the authors find only minimal effects of loss of Rela on HSC function, perhaps due to the use of an inducible system in the case of Laranjeira et al and constitutive Rela loss in the Stein and Baldwin article.5 This raises the question of the source of these differences and the potential need to mitigate the mild but notable hematological effects of Rela and miR-146a perturbation in normal controls.
Another compelling question is the link between miR-146a, MPN, and autoimmunity. miR-146a-null mice have elevated inflammatory cytokines, myeloid proliferation, splenomegaly, and abnormal T cells, and they develop autoimmunity.8 Given the coexistence of MPN and autoimmune disease,9,10 this suggests dysregulation of the NF-ĸB pathway in MPN could be contributing to autoimmunity. Intriguingly, CA-4948 appears to be more effective against diseases with a preference for the long isoform IRAK4-L vs short IRAK4-S. What determines the ratio of short and long isoforms of IRAK4 in MPN? Investigating this partiality could assist in validating or maximizing the efficacy of IRAK inhibitors in MPN. Additionally, the reductions in colony formation from dual inhibition of JAK2 and IRAK4 warrant further in vivo experimentation on the antineoplastic benefits of simultaneously targeting these mechanisms.
Discovery of the JAK2, MPL, and CALR driver mutations changed the landscape of MPN research, but the unresolved need for a cure and more effective therapies leads us to wonder what other factors are at play. This study takes a significant step forward in establishing NF-ĸB as a bona fide target in MPN to not only address chronic inflammation but also ameliorate clonal expansion and fibrosis.
Conflict-of-interest disclosure: The authors declare no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal