Visual Abstract
Arterial and venous thromboses are classically considered distinct disease states, with arterial thrombosis mediated predominantly by platelets and therefore, treated with antiplatelet therapy, and venous thrombosis mediated by the plasmatic coagulation system and treated with anticoagulation. However, co-occurrence of arterial and venous events is common, and there is increasing evidence of shared risk factors and pathophysiologic overlap. This presents a management challenge: does the patient with venous and arterial thrombosis, require anticoagulation, antiplatelet therapy, or both? Herein, we present a structured approach to the evaluation and management of patients with venous thrombosis who are also at risk for or have a history of an arterial thromboembolic event. We emphasize the importance of defining the indications for antithrombotic therapy, as well as the evaluation of factors that influence both thrombotic and bleeding risk, including disorder-specific and patient-specific factors, as well as the inherent risk balance of antithrombotic therapy regimens. We illustrate this approach in 4 cases, discussing the unique considerations and recent updates in the management of venous thrombosis, acute noncardioembolic ischemic stroke, coronary artery disease and acute myocardial infarction, and peripheral artery disease after revascularization.
Background
The pathophysiology and treatment of venous and arterial thrombosis have classically been distinct. Management of venous thromboembolism (VTE) has focused on decreasing coagulation factor activity with anticoagulation and arterial thrombosis on inhibiting platelets with antiplatelet therapy.1 However, there is increasing recognition of the interplay between the 2. Patients with arterial thrombosis are at an increased risk of VTE,2,3 and patients with VTE are at an increased risk for atherosclerosis and arterial thrombosis.4-7 This is likely due to shared risk factors including components of metabolic syndrome (obesity, hypercholesterolemia, diabetes mellitus, and hypertension),8,9 cigarette smoking, older age, malignancy, and others.10 Concurrent risk may also be due to interconnectivity of platelet-mediated and coagulation-mediated thrombotic pathways. Antiplatelet therapy has some ability to prevent VTE, as demonstrated by its use postorthopedic surgery,11 and there is increasing recognition that anticoagulation can prevent arterial thrombosis.12-14
Given this interplay, providers frequently manage antithrombotic treatment in patients who require targeting both the arterial and venous vasculature. The inclination is to simply add therapies, combining anticoagulation with antiplatelet therapy or vice versa. This may be appropriate in some cases, but in others may increase bleeding risk without decreasing thrombotic risk, the latter of which is most clearly demonstrated from literature on concurrent anticoagulation and antiplatelet therapy in patients with atrial fibrillation and acute coronary syndrome.15-17 However, thrombotic and bleeding risk are challenging to quantify, as few studies have evaluated patients with multiple indications for antithrombotic therapy beyond the atrial fibrillation population. Furthermore, because of the paucity of data, published guidance from scientific societies may differ, particularly in various patient subgroups. Therefore, the use of best available evidence, collective interpretation of guidelines, and extrapolation from similar clinical scenarios is often required.
Agent selection is also challenging because antithrombotic therapy strategies vary significantly based on the indication for use. There is unique terminology for combination antithrombotic therapy in some specialties (Table 1), and dosing of direct oral anticoagulants (DOACs) is highly dependent on indication for use (Table 2).
Terminology for antithrombotic therapy regimens and indications in venous and arterial thrombosis
| Term . | Definition . | Common applications . |
|---|---|---|
| Antithrombotic therapy regimens | ||
| Dual antiplatelet therapy | Low dose aspirin + P2Y12 inhibitor∗ | Any arterial thrombosis |
| Dual antithrombotic therapy | Anticoagulation + 1 antiplatelet agent | Atrial fibrillation + CAD |
| Dual pathway inhibition | Low dose anticoagulation (rivaroxaban 2.5 mg daily) + antiplatelet agent(s) | Stable CAD PAD after revascularization |
| Triple antithrombotic therapy | Anticoagulation + 2 antiplatelet agents | Atrial fibrillation + CAD |
| Triple therapy | ||
| Indications for antithrombotic therapy | ||
| Prevention of stent thrombosis84 | CAD, PAD | |
| Early | 0-30 d after stent implantation | |
| Late | >30 d to 1 y after stent implantation | |
| Very late | >1 y after stent implantation | |
| Initiation phase | Initial provision of anticoagulants after VTE diagnosis (7 d for apixaban; 21 d for rivaroxaban)35 | VTE |
| Treatment phase | Period after initiation to complete treatment for acute VTE (3 mo)35 | |
| Extended phase | Period of anticoagulant at full or reduced dose for secondary prevention (after 3 mo)35 | |
| Term . | Definition . | Common applications . |
|---|---|---|
| Antithrombotic therapy regimens | ||
| Dual antiplatelet therapy | Low dose aspirin + P2Y12 inhibitor∗ | Any arterial thrombosis |
| Dual antithrombotic therapy | Anticoagulation + 1 antiplatelet agent | Atrial fibrillation + CAD |
| Dual pathway inhibition | Low dose anticoagulation (rivaroxaban 2.5 mg daily) + antiplatelet agent(s) | Stable CAD PAD after revascularization |
| Triple antithrombotic therapy | Anticoagulation + 2 antiplatelet agents | Atrial fibrillation + CAD |
| Triple therapy | ||
| Indications for antithrombotic therapy | ||
| Prevention of stent thrombosis84 | CAD, PAD | |
| Early | 0-30 d after stent implantation | |
| Late | >30 d to 1 y after stent implantation | |
| Very late | >1 y after stent implantation | |
| Initiation phase | Initial provision of anticoagulants after VTE diagnosis (7 d for apixaban; 21 d for rivaroxaban)35 | VTE |
| Treatment phase | Period after initiation to complete treatment for acute VTE (3 mo)35 | |
| Extended phase | Period of anticoagulant at full or reduced dose for secondary prevention (after 3 mo)35 | |
CAD, coronary artery disease; PAD, peripheral artery disease.
P2Y12 inhibitors: clopidogrel, prasugrel, ticagrelor, and cangrelor.
Doses, terminology, and indications for oral factor Xa and oral direct thrombin inhibitors
| Term . | Dose . | Indications . | Notes . |
|---|---|---|---|
| Initiation dose | Rivaroxaban 15 mg bid × 21 d | VTE | Start therapeutic dose after indicated duration |
| Apixaban 10 mg bid × 7 d | |||
| Therapeutic dose | Rivaroxaban 20 mg daily | 1. VTE 2. NVAF | For VTE, dose initiated after at least 5 d of parenteral anticoagulation§ |
| Edoxaban 60 mg daily§ | |||
| Dabigatran 150 mg bid§ | |||
| Apixaban 5 mg bid | |||
| Adjusted dose | Rivaroxaban 15 mg daily | NVAF | CrCl 15-50 mL/min |
| Edoxaban 30 mg daily | 1. VTE 2. NVAF | 1. CrCl 15-50 mL/min, weight ≤60 kg, certain concomitant P-gp inhibitors 2. CrCl 15-50 mL/min | |
| Apixaban 2.5 mg bid | NVAF | 2 of 3 criteria met: SCr, ≥1.5 mg/dL; age ≥80 y; weight, ≤60 kg | |
| Low dose | Apixaban 2.5 mg bid∗†‡ | VTE | 1. Primary prevention: VTE prevention in patients with cancer,∗ THA,† and TKA† 2. Secondary prevention: extended phase therapy for patients with VTE‡ |
| Rivaroxaban 10 mg daily∗†‡ | |||
| Dabigatran 110 mg 1-4 h after surgery, then 220 mg daily for 28-35 d† | |||
| Very low dose | Rivaroxaban 2.5 mg bid | 1. PAD + revascularization within 10 d 2. Stable CAD or PAD | With low dose aspirin |
| Term . | Dose . | Indications . | Notes . |
|---|---|---|---|
| Initiation dose | Rivaroxaban 15 mg bid × 21 d | VTE | Start therapeutic dose after indicated duration |
| Apixaban 10 mg bid × 7 d | |||
| Therapeutic dose | Rivaroxaban 20 mg daily | 1. VTE 2. NVAF | For VTE, dose initiated after at least 5 d of parenteral anticoagulation§ |
| Edoxaban 60 mg daily§ | |||
| Dabigatran 150 mg bid§ | |||
| Apixaban 5 mg bid | |||
| Adjusted dose | Rivaroxaban 15 mg daily | NVAF | CrCl 15-50 mL/min |
| Edoxaban 30 mg daily | 1. VTE 2. NVAF | 1. CrCl 15-50 mL/min, weight ≤60 kg, certain concomitant P-gp inhibitors 2. CrCl 15-50 mL/min | |
| Apixaban 2.5 mg bid | NVAF | 2 of 3 criteria met: SCr, ≥1.5 mg/dL; age ≥80 y; weight, ≤60 kg | |
| Low dose | Apixaban 2.5 mg bid∗†‡ | VTE | 1. Primary prevention: VTE prevention in patients with cancer,∗ THA,† and TKA† 2. Secondary prevention: extended phase therapy for patients with VTE‡ |
| Rivaroxaban 10 mg daily∗†‡ | |||
| Dabigatran 110 mg 1-4 h after surgery, then 220 mg daily for 28-35 d† | |||
| Very low dose | Rivaroxaban 2.5 mg bid | 1. PAD + revascularization within 10 d 2. Stable CAD or PAD | With low dose aspirin |
The symbols in the “Dose” column correspond to those in the “Notes” column.
bid, twice a day; CrCl, creatinine clearance; NVAF, nonvalvular atrial fibrillation; SCr, serum creatinine; THA, total hip arthroplasty; TKA, total knee arthroplasty.
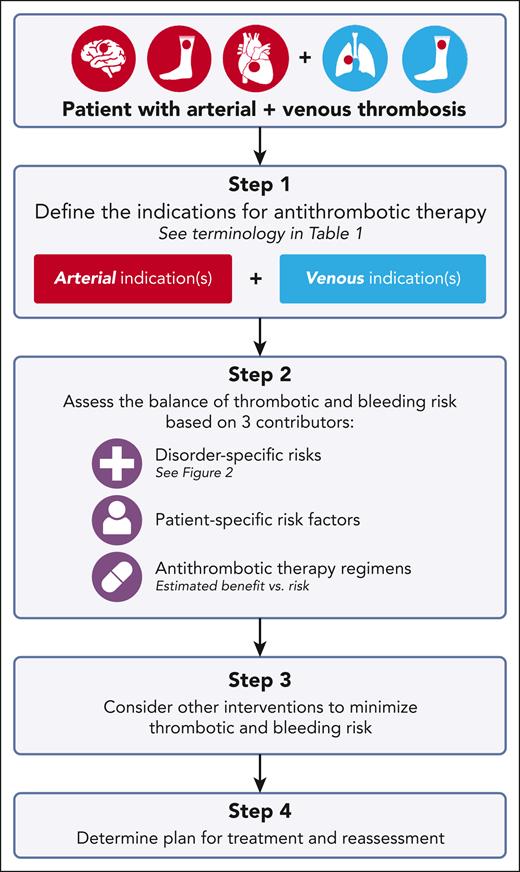
Herein, we present a structured approach to the selection of antithrombotic therapy for patients with extremity deep vein thrombosis (DVT) and/or pulmonary embolism (PE) with known atherosclerosis or a history of arterial thrombosis (Figure 1). Unusual site venous thrombosis (eg, splanchnic and cerebral) are not addressed given limited data on management for these sites independent of concurrent arterial events. We apply this framework to 4 cases, highlighting existing guidance on antithrombotic therapy selection in specific clinical scenarios. We also discuss common management themes, including defining therapy indications and duration, and assessing the balance of thrombotic and bleeding risk. It is important to note that this article cannot address all scenarios of overlap of venous and arterial thrombosis, nor can it address the nuances of individualized patient care, but we intend to provide a framework upon which the care of these complex patients can be developed.
Structured approach to antithrombotic therapy selection in patients with venous and arterial thrombosis.
Structured approach to antithrombotic therapy selection in patients with venous and arterial thrombosis.
Clinical approach
Step 1: Define the indication(s) for antithrombotic therapy
Defining indication(s) for antithrombotic therapy assigns a clear purpose to treatment, serving as a necessary foundation for optimal antithrombotic agent(s) selection. Table 1 represents frequently used terminology for specific disorders.
For example, in patients with coronary artery disease (CAD) treated with percutaneous coronary intervention (PCI), the initial indication for antithrombotic therapy is prevention of in-stent thrombosis. The risk of in-stent thrombosis varies over time, with early, late, and very late phases (Table 1). Long-term, patients may require antithrombotic therapy for prevention of both in-stent thrombosis and future myocardial infarction. In contrast, in patients with acute DVT and/or PE, the initial indication for anticoagulation is treatment of thrombosis, and based on the anticoagulant selected, a different dose may be required for initiation and treatment phases (Tables 1 and 2). After treatment phase, the indication for anticoagulation is prevention of recurrent VTE.
Understanding the indication for antithrombotic therapy, particularly when a patient has multiple indications and when indications change over time, allows for an accurate characterization of thrombotic risk necessary for therapy selection.
Step 2: Assess the balance of thrombotic and bleeding risk
Once the indications for antithrombotic therapy are defined, thrombotic and bleeding risk are assessed. Thrombotic risk was evaluated first: what is the risk of a recurrent thrombotic event and how much does a given therapy decrease that risk? The potential decrease in thrombotic risk was then weighed against bleeding risk, with the goal to identify the optimal risk balance for the individual. Three major contributors that influence thrombotic and bleeding risks are: (1) disorder-specific risk patterns, (2) patient-specific risk factors, and (3) antithrombotic therapy regimens.
Disorder-specific risk patterns
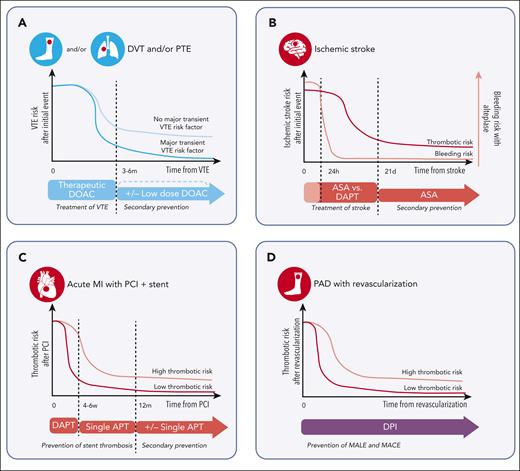
Figure 2 includes illustrations of how disorder-specific patterns influence antithrombotic therapy selection, providing a visual representation of the natural history of 4 thrombotic conditions: VTE, acute myocardial infarction after PCI, peripheral artery disease (PAD) after revascularization, and acute ischemic stroke. The dynamic changes in risk over time for each disorder are discussed in the cases section.
Illustration of the thrombotic risk and antithrombotic treatment approach for common thrombotic conditions. Diagnoses include (A) VTE, (B) ischemic stroke, (C) acute myocardial infarction with percutaneous coronary intervention and stent placement, and (D) PAD with revascularization. Red circles represent an arterial thrombotic event of the pictured organ and blue circles represent a venous thrombotic event. Time on the x-axis is approximated and not to scale, with time 0 representing the occurrence of the thrombotic event. Antithrombotic therapies are distinguished by colors, with red representing antiplatelet therapy, blue representing anticoagulation, and purple representing combination of anticoagulation and antiplatelet therapy. APT, antiplatelet therapy; ASA, aspirin; MI, myocardial infarction.
Illustration of the thrombotic risk and antithrombotic treatment approach for common thrombotic conditions. Diagnoses include (A) VTE, (B) ischemic stroke, (C) acute myocardial infarction with percutaneous coronary intervention and stent placement, and (D) PAD with revascularization. Red circles represent an arterial thrombotic event of the pictured organ and blue circles represent a venous thrombotic event. Time on the x-axis is approximated and not to scale, with time 0 representing the occurrence of the thrombotic event. Antithrombotic therapies are distinguished by colors, with red representing antiplatelet therapy, blue representing anticoagulation, and purple representing combination of anticoagulation and antiplatelet therapy. APT, antiplatelet therapy; ASA, aspirin; MI, myocardial infarction.
Patient-specific risk factors
Many patient-specific factors influence the thrombosis-bleeding risk balance, some linked to the thrombotic disorder and some independent of it. For example, in patients with coronary artery stent placement, the risk of in-stent thrombosis varies based on patient-related, stent-related, and procedure-related factors.18 Patients with acute ischemic stroke are at risk for hemorrhagic transformation of the infarct, a risk that is influenced by the use of tPA, the need for anticoagulation, and patient-related factors including age, cardiovascular risk factors, and infarct volume.19
Multiple scoring systems exist that attempt to quantify patient-specific bleeding risk in patients with venous20,21 and arterial22,23 thrombosis. These scores are not validated in patients with multiple thrombotic conditions, but evaluation of the components they include (eg, renal function and blood pressure) can guide individualized risk assessment and management. Other modifiable bleeding risk factors to investigate include concurrent medications (eg, nonsteroidal anti-inflammatory drugs) and fall risk.
Antithrombotic therapy regimens
Inherent to all antithrombotic therapy regimens, is a tradeoff between decreasing thrombotic risk and increasing bleeding risk. Evaluation of available data for a given site of thrombosis is necessary to determine which regimen(s) strike(s) the optimal balance. Importantly, there are often gaps in the literature and a patient’s exact antithrombotic therapy indication(s) is/are not addressed, so extrapolation from best available evidence is necessary. Multidisciplinary collaboration is also required, as providers with expertise in the arterial vasculature (eg, neurology, cardiology, and vascular surgery) may not have expertise in the venous vasculature and vice versa (eg, hematology and pulmonology).
Step 3: Consider other interventions to minimize thrombotic and bleeding risk
Although focus is often on antithrombotic therapy selection, other treatment strategies influence thrombotic and bleeding risk. For example, in patients with atherosclerosis–mediated arterial thrombosis, addressing atherosclerotic risk factors is essential to prevent recurrence. In addition, modifiers of bleeding risk should be considered, such as the use of proton pump inhibitors (PPIs) for the prevention of gastrointestinal bleeding (discussed in case 2).
Step 4: Determine a plan for treatment and reassessment
With consideration of the indications for treatment, along with the incorporation of disorder-related, patient-specific, and antithrombotic therapy–related thrombotic and bleeding risk, a comprehensive treatment plan is developed. Importantly, this is not a one-time development, as risks change over time due to both disorder-specific (Figure 2) and patient-related factors, so therapy determinations should be made concurrently with a plan to reassess.
Cases and discussion
Case 1
A 58-year-old woman with a history of hypertension, hyperlipidemia, type 2 diabetes mellitus, and obesity shows a new proximal DVT without major transient risk factors. She takes aspirin 81 mg daily for prevention of CAD.
Considerations
Should aspirin be used for primary prevention of atherosclerotic cardiovascular disease (ASCVD)?
The use of aspirin for primary prevention of ASCVD has a convoluted history, with multiple large, randomized trials producing conflicting results over 40 years. More recent studies have highlighted the limited efficacy of aspirin for primary prevention and its potential harm due to bleeding.24 As a result, recent guidelines recommend consideration of aspirin for primary prevention only in younger patients (with varying age thresholds ranging from <60 years to 70 years) with higher ASCVD risk and recommend against use in those with additional factors that increase bleeding risk, including older age and concurrent anticoagulation.25,26 However, despite these recommendations, co-prescription of aspirin for this indication with anticoagulation continues.27,28
If aspirin is stopped, does anticoagulation alone help prevent ASCVD?
Although anticoagulation has not been directly evaluated for primary prevention of ASCVD, available data support that it has activity in the arterial vasculature. Pathophysiologic studies suggest DOACs prevent progression of coronary atherosclerosis.29,30 In patients with atrial fibrillation and stable coronary disease, rivaroxaban monotherapy was noninferior to its combination with a single antiplatelet agent in the prevention of arterial events and death.31 In addition, interventions to decrease unnecessary coprescribing of aspirin with anticoagulation, have decreased bleeding rates without increasing thrombotic complications.32
Antithrombotic treatment and secondary prevention of VTE
The natural history and use of antithrombotic therapy in VTE is presented in Figure 2A. There is initially a high thrombotic risk, so therapeutic dose anticoagulation is required. Treatment for acute VTE with oral factor Xa inhibitors includes an initial higher intensity, “initiation dose” (apixaban 10 mg twice daily for 7 days and rivaroxaban 15 mg twice daily for 21 days), followed by continuation of a therapeutic dose (apixaban 5 mg twice daily and rivaroxaban 20 mg daily) (Table 2). Dabigatran and edoxaban require parental anticoagulation for an initiation period of at least 5 days before oral therapeutic dose anticoagulation. After 3 to 6 months, thrombotic risk declines to a level dependent on whether an event was provoked by a major transient risk factor or not. If the event was provoked, the baseline risk is low so no secondary prevention is required and anticoagulation is stopped. If the event was without a major transient risk factor (ie, unprovoked or with a persistent major risk factor), anticoagulation is continued to prevent recurrent VTE. Risk stratification strategies (eg, D-dimer, prediction models, or thrombophilia testing) can sometimes be used to determine if a patient has a lower recurrence risk and could stop anticoagulation,33,34 but we will assume continued anticoagulation in this manuscript for clarity of discussion.
For extended phase management of VTE (ie, secondary prevention), guidelines support the use of low dose apixaban (2.5 mg twice daily) or rivaroxaban (10 mg daily).35,36 These recommendations are based on 2 trials that compared both therapeutic and low dose oral Xa inhibitors with a comparator (placebo for apixaban37 and aspirin for rivaroxaban38). In both studies, the efficacy of therapeutic and low dose oral Xa inhibitors were superior to their comparator. Notably, guidelines define the extended phase of VTE treatment starting 3 months after VTE,35 but the aforementioned trials evaluated transition to low dose at 6 months (Figure 1A). Therefore, we favor assessing for dose reduction at 6 months unless there is significant concern for bleeding, in which case earlier dose reduction can be considered.
Structured assessment
Step 1
The patient has multiple atherosclerotic risk factors but has never had an arterial event, so her indication for aspirin is primary prevention of ASCVD. She now requires anticoagulation for the treatment of DVT. Given the VTE was unprovoked, she requires long-term anticoagulation for secondary prevention.
Step 2
The use of aspirin for primary prevention of ASCVD in this patient can be considered per guidelines given her younger age and high ASCVD risk. However, she now requires anticoagulation, and because the potential benefit of aspirin in decreasing ASCVD risk is outweighed by bleeding risk with concurrent use of anticoagulation and aspirin, guidelines recommend aspirin cessation.25,26 Although the efficacy of anticoagulation in primary prevention of ASCVD has not been evaluated, available data suggest it provides some protection from progressive atherosclerosis and arterial thrombotic events.
Step 3
The patient has multiple ASCVD risk factors and would benefit from interventions such as statin use, weight loss, and diabetes management. These interventions may also decrease VTE risk, as statin use has been shown to decrease the risk of both incident39 and recurrent VTE.40 Weight gain increases the risk of initial VTE,41 but the influence of weight loss to decrease recurrent VTE has not been studied, although obesity itself does not appear to increase recurrent VTE risk.42,43
Step 4
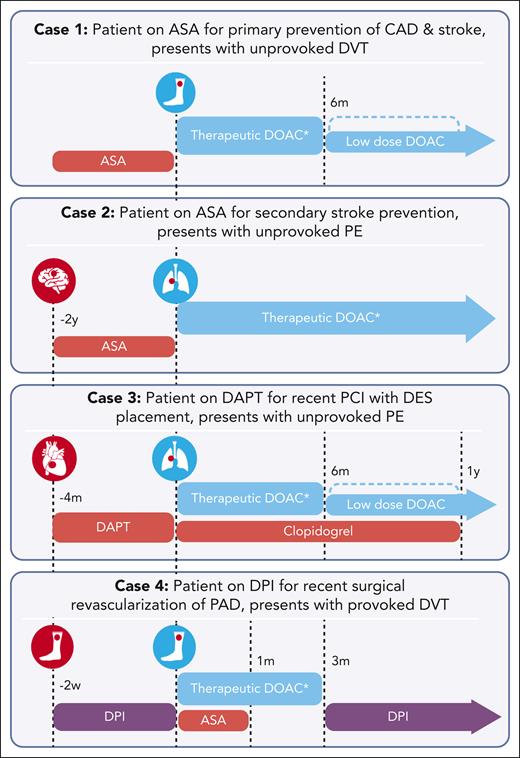
Figure 3 presents the patient’s management plan, along with the plan for all subsequent cases. She starts initiation dose apixaban 10 mg twice daily for 7 days followed by a therapeutic dose of 5 mg twice daily. We recommend aspirin cessation, discuss strategies for weight loss and diabetes management, and encourage use of statin and antihypertensive therapy. Close follow-up is arranged with primary care and hematology, with consideration to reduce apixaban to 2.5 mg twice daily at 6 months, along with continued management of ASCVD risk factors. We recommend continued aspirin cessation even after apixaban dose reduction because of the concern that bleeding risk continues to outweigh any small benefit in primary ASCVD prevention.
Timeline of antithrombotic therapy for discussed cases. For each case, the red circle represents the arterial thrombotic event, and the blue circle represents the venous thrombotic event. Time indicators reflect occurrence of events relative to the venous thrombotic event. Antithrombotic therapies are distinguished by colors, with red representing antiplatelet therapy, blue representing anticoagulation, and purple representing a combination of anticoagulation and antiplatelet therapy. ∗For all patients receiving therapeutic dose oral Xa inhibitors for venous thrombosis, an initiation dose is recommended (per package insert, and presented in Table 1). ASA, aspirin.
Timeline of antithrombotic therapy for discussed cases. For each case, the red circle represents the arterial thrombotic event, and the blue circle represents the venous thrombotic event. Time indicators reflect occurrence of events relative to the venous thrombotic event. Antithrombotic therapies are distinguished by colors, with red representing antiplatelet therapy, blue representing anticoagulation, and purple representing a combination of anticoagulation and antiplatelet therapy. ∗For all patients receiving therapeutic dose oral Xa inhibitors for venous thrombosis, an initiation dose is recommended (per package insert, and presented in Table 1). ASA, aspirin.
Case 2
A 75-year-old man with a history of noncardioembolic ischemic stroke 2 years prior, on aspirin 81 mg for secondary stroke prevention, presents with PE without major transient risk factors.
Considerations
Antithrombotic therapy selection in ischemic stroke
In acute noncardioembolic ischemic stroke, there is an initial high thrombotic risk but is unique from coronary and PAD, there is also a high bleeding risk because of potential hemorrhagic transformation (Figure 2B). Therefore, antithrombotic therapy is often held for the first 24 hours, particularly if thrombolytics are given, only starting when the hemorrhage risk declines.44 Initial dual antiplatelet therapy (DAPT) may be considered after an acute event in select patients (minor stroke, high-risk transient ischemic attack, and severe symptomatic intracranial stenosis), but DAPT beyond 90 days is not recommended because of lack of benefit and increased hemorrhage risk.45 Antiplatelet therapy is often continued indefinitely for secondary stroke prevention with either aspirin (50-325 mg daily), clopidogrel (75 mg daily), or aspirin (25 mg) with extended-release dipyridamole (200 mg twice daily).46
Routine use of anticoagulation for secondary stroke prevention in noncardioembolic stroke is not recommended because of comparable efficacy to antiplatelet therapy but increased bleeding risk. Previous studies investigated vitamin K antagonists vs aspirin for secondary prevention, with analysis favoring aspirin because of similar efficacy in stroke prevention but lower bleeding risk.47 Furthermore, in patients with embolic strokes of undetermined source, a comparison of rivaroxaban 15 mg daily vs aspirin 100 mg daily demonstrated similar rates of recurrent stroke, although the former was associated with increased incidence of bleeding.48 These findings suggest that although anticoagulation is not preferred for secondary stroke prevention because of increased bleeding risk, if a patient requires anticoagulation for another indication, it provides protection against recurrent stroke. Importantly, the role of oral factor Xa inhibitors in patients with an established atherosclerotic mechanism (as opposed to those who met the criteria for embolic strokes of undetermined source) has not been evaluated. Nevertheless, existing guidelines recommend stopping antiplatelet therapy in a patient with new VTE unless the patient has high arterial thrombotic risk with low bleeding risk.49 Although dose reduction of oral Xa inhibitors can be considered 3 to 6 months after VTE as outlined in case 1, we did not reduce the dose in patients with average bleeding risk and a concurrent arterial indication given the lack of data on the use of low dose for arterial indications. However, given the emerging data on the use of very low dose rivaroxaban with antiplatelet therapy for arterial indications (discussed in case 4), we may consider dose reductions in the future pending additional research.
Use of PPIs to prevent gastrointestinal bleeding
The use of PPIs in patients on antithrombotic therapy for primary prevention of gastrointestinal bleeding is debated. A retrospective evaluation of patients receiving anticoagulation indicated a lower risk of hospitalization for gastrointestinal bleeding in those receiving PPIs.50 In addition, a recent meta-analysis demonstrated lower rates of upper gastrointestinal bleeding in patients prescribed oral anticoagulants and PPIs, with greatest benefit in patients with higher bleeding risk, including those receiving concurrent aspirin.51 Investigation of PPI use in patients on very low dose rivaroxaban and/or aspirin for stable CAD demonstrated no reduction in upper gastrointestinal bleeding events, perhaps owinge to the lower anticoagulation dose used in this study.52 Available guidelines in cardiology recommend use of PPIs in either patients on DAPT53 or patients on 2 or more antithrombotic agents.49
Structured assessment
Step 1
The patient is currently taking aspirin for secondary prevention of ischemic stroke, and now requires anticoagulation for the treatment of PE. No major transient risk factors were identified, so he requires long-term anticoagulation for secondary prevention of VTE.
Step 2
Based on disorder-specific literature, the concurrent use of anticoagulation and antiplatelet therapy compared with anticoagulation alone for secondary stroke prevention would provide similar efficacy but increase bleeding risk. The patient reports a history of peptic ulcer disease and is noted to have elevated blood pressure during hospitalization. His VTE-BLEED score54 is 4 and his HAS-BLED score55 is 5 (noting that the HAS-BLED score is validated in patients with atrial fibrillation, which this patient does not have), both indicating a high risk for bleeding. The case was also discussed with the patient’s neurology providers.
Step 3
We discussed optimization of blood pressure control with the patient’s primary care provider, as well as starting a PPI given a history of peptic ulcer disease and the need for antithrombotic therapy.
Step 4
The patient was started on anticoagulation with initiation dose rivaroxaban 15 mg twice daily. Aspirin was stopped due to concern that any benefit in secondary stroke prevention is outweighed by bleeding risk. Rivaroxaban was decreased to therapeutic dose 20 mg daily after 21 days, with a plan to continue this dose long-term rather than decreasing to 10 mg daily given no data on low dose for secondary stroke prevention. He also starts additional antihypertensives and a PPI. Close follow-up was scheduled for continued reassessment of risk and management of comorbidities with primary care, hematology, and neurology.
Case 3
A 59-year-old woman with a recent history of myocardial infarction requiring PCI with drug eluting stent (DES) placement 4 months prior, on DAPT, presents with PE without major transient risk factors.
Considerations
Antithrombotic therapy selection after PCI
Initial treatment of patients is focused on prevention of stent thrombosis (Figure 2C). In the past, bare metal stents required shorter duration of DAPT compared with first-generation DES56 because of increased incidence of late stent thrombosis with the latter.57 However, newer generation DES have lower rates of late and very late stent thrombosis so shorter duration of DAPT is required and bare metal stents are no longer routinely used.49,53
DAPT is used initially after PCI due to risk of early stent thrombosis (0-30 days after stent implantation). The duration of DAPT in patients at high bleeding risk, including those on anticoagulation, has been primarily explored in patients with atrial fibrillation and acute coronary syndrome, but this data is commonly extrapolated to patients with VTE.49 Guidance documents49,53,58-60 and a recent clinical trial61 support limited duration triple antithrombotic therapy (TAT; anticoagulation plus DAPT; Table 1) of 4 to 6 weeks after PCI, followed by transition to dual antithrombotic therapy (DAT; anticoagulation plus a P2Y12 inhibitor; Table 1).49,53,58-60 Longer duration TAT is only used in patients with very high risk of stent thrombosis. However, assessing risk of stent thrombosis is complex given multiple patient-specific, stent-specific, and procedure-specific factors contribute.18 There is ongoing debate if even shorter durations (1 week) of TAT can be considered in low risk patients.62
Which anticoagulant(s) and antiplatelet agent(s) should be used?
When concurrent anticoagulation and antiplatelet therapy is required, DOACs are preferred over warfarin based on extrapolation from guidelines in patients with cardiac disease,49,58 and based on guidelines supporting these anticoagulants over warfarin for VTE.35,36 These recommendations are also supported by existing data suggesting a lower bleeding risk compared with vitamin K antagonists as single agents63 and in combination with antiplatelet therapy.64,65 There are certain scenarios when warfarin may be preferred or required for thrombosis prevention (eg, antiphospholipid syndrome and mechanical mitral valve replacement), in which case a potential increase in bleeding risk with concurrent antiplatelet therapy must be acknowledged.
In patients requiring TAT, guidelines recommend clopidogrel over ticagrelor in combination with aspirin due to increased bleeding risk with ticagrelor, noting that comparison trials have incompletely evaluated efficacy in thrombosis prevention.49,58,59 When TAT is de-escalated, aspirin is preferably stopped rather than clopidogrel because of presumed increased efficacy of the latter, although direct comparison of these regimens has not been performed.49,59
Structured assessment
Step 1
The patient is currently taking DAPT for prevention of late stent thrombosis, and now requires anticoagulation for the treatment of PE. The VTE was unprovoked, so she requires long-term anticoagulation for secondary prevention of VTE.
Step 2
A thorough history and physical examination did not identify any modifiable comorbidities that influence her thrombotic or bleeding risk. Based on the review of available literature, the use of TAT vs DAT would not provide clear benefit in prevention of late stent thrombosis and would increase bleeding risk. Her care was also discussed with cardiology.
Step 3
Step 4
For prevention of late stent thrombosis, aspirin was stopped and clopidogrel continued for a planned duration of 1 year. For treatment and secondary prevention of VTE, she receives apixaban 10 mg twice daily for 7 days and then 5 mg twice daily. A PPI was also started. We arranged for close follow-up with hematology and cardiology and plan to decrease apixaban to 2.5 mg twice daily after 6 months.
Case 4
A 71-year-old man underwent endovascular revascularization/stenting for PAD 2 weeks before presentation and was treated postprocedure with rivaroxaban 2.5 mg twice daily and aspirin 81 mg daily. He now presents with proximal lower extremity DVT.
Considerations
Antithrombotic therapy in PAD
Patients with PAD are at an increased risk of both major adverse limb events (MALE; including major amputation and acute limb ischemia) and major adverse cardiovascular events (MACE; including myocardial infarction, ischemic stroke, and cardiovascular death). Treatment often includes interventional strategies for revascularization and/or antithrombotic therapy to prevent these outcomes (Figure 1D). There is a paucity of trials to guide antithrombotic therapy selection, particularly after revascularization, so existing guidelines provide recommendations with low level evidence.49,66-68
In patients with PAD without recent revascularization and a concurrent indication for anticoagulation (eg, unprovoked VTE), anticoagulation without antiplatelet therapy is recommended.49,67 If a patient on anticoagulation requires revascularization, guidance has generally recommended anticoagulation alone after surgical revascularization and anticoagulation with antiplatelet therapy for a limited duration (1-3 months) after endovascular revascularization.49,67 However, this decision is influenced by patient-specific factors, procedure-specific factors, and intraprocedural findings that may increase thrombotic and/or bleeding risk, and there is no consensus on how to define higher risk patients.69
Dual pathway inhibition (DPI)
Two pivotal clinical trials introduced a new antithrombotic regimen, rivaroxaban 2.5 mg twice in combination with aspirin 75-100mg daily, in stable CAD and symptomatic PAD after revascularization.12,13 The regimen is referred to as DPI (Table 1) because of targeting both platelet function and coagulation.
Interestingly, both trials with DPI, demonstrated a decrease in both arterial and venous thrombosis,14,70,71 with particular benefit in patients with PAD with high risk of DVT after both surgical72 and endovascular73 revascularization. Importantly, both trials demonstrated a small increase in bleeding risk of DPI compared with aspirin alone, but there was no difference in intracranial or fatal bleeding.12,13
The changing landscape of oral Xa inhibitor dosing
DPI introduces yet another dose of oral Xa inhibitors to an already complex dosing landscape (Table 2). The existence of different dosing strategies highlights the unique balance of thrombotic and bleeding risk for different anatomic sites of thrombosis, and the importance of defining the intent of therapy to select the appropriate dose. Importantly, the use of dosing strategies outside of studied indications is common in clinical practice and has been shown to adversely affect patient outcomes.74-78 The existence of multiple dosing options also introduces potential for prescribing error,79-81 so system level strategies including computerized clinical decision support tools and/or anticoagulation stewardship programs are increasingly important.82,83
Structured assessment
Step 1
The patient is currently taking DPI after endovascular stenting for prevention of MALE and MACE. His DVT was provoked by 2 major transient risk factors: his procedure and subsequent hospitalization. Therefore, an anticoagulant treatment phase of 3 months was considered appropriate, without a need for secondary prevention of VTE.
Step 2
The case was discussed with vascular surgery, who assesses the patient to be at high risk for MALE given endovascular revascularization within the last month. Thorough history, physical examination, and laboratory data did not identify any patient-specific risk factors for bleeding. Guidelines support the use of anticoagulation with antiplatelet therapy for a limited duration (1 month) after endovascular revascularization in patients with low bleeding risk.67
Step 3
Concurrent use of anticoagulation and antiplatelet therapy warrants consideration of a PPI. The patient will also require comprehensive evaluation for exercise therapy and risk factor modification for long-term prevention of MACE/MALE.
Step 4
For the treatment of VTE, rivaroxaban was increased from very low dose 2.5 mg to 15 mg twice daily for 21 days, with plan to decrease to 20 mg daily after that time. Given the concern for risk of MALE in a patient with low bleeding risk, aspirin 81 mg was continued. Close follow-up was arranged, with a plan to stop aspirin 1 month after stenting. After 3 months of therapeutic anticoagulation for provoked DVT, rivaroxaban will be reduced back to 2.5 mg twice daily and aspirin will be restarted. A PPI was initiated with a plan to discontinue when antithrombotic therapy is deescalated back to very low dose rivaroxaban and aspirin, based on the lack of clear decrease in bleeding risk with PPI use and this antithrombotic regimen.52
Conclusion
In conclusion, because of shared thrombosis risk factors and pathophysiologic overlap, the co-occurrence of venous and arterial thrombosis is common. Selecting optimal antithrombotic therapy for patients with venous thrombosis who also have an arterial thrombosis or atherosclerotic disease can be challenging. The inclination is often to add therapies, combining both anticoagulation and antiplatelet therapy, but this approach is often without demonstrated benefit and associated with significant bleeding risk. There is growing evidence of the benefit of anticoagulation on arterial vascular bed thrombosis, which supports recommendations for de-escalation of antiplatelet therapy when patients require anticoagulation. A structured approach, including clear definitions of indications for therapy and assessment of disorder-specific, patient-specific, and antithrombotic therapy–related thrombotic and bleeding risk, is important to develop the optimal management strategy for thrombosis prevention in patients with both venous and arterial disease.
Note added in proof
Since submission of the manuscript, 2 updated society guidelines that provide further details regarding the limited duration of TAT for patients who have an indication for anticoagulation (specifically atrial fibrillation) and undergo PCI have been published. In case 3, we highlight available guidance that supported TAT for 4 to 6 weeks after PCI and state that there is ongoing debate as to whether even shorter durations (1 week) of TAT might be appropriate for low-risk patients. New guidelines now support a much stronger call for only 1 week of TAT. Per the American College of Cardiology, American Heart Association, American College of Chest Physicians, and Heart Rhythm Society, early discontinuation of aspirin (after 1 to 4 weeks) and continuation of DAT with a DOAC and a P2Y12 inhibitor are preferred over longer-duration TAT.85 Similarly, the European Society of Cardiology recommends up to 1 week of TAT followed by DAT, preferably with clopidogrel and a DOAC.86 Both acknowledge that patients at higher risk of stent thrombosis may warrant longer-duration TAT (up to 4 weeks).
Given the constant accumulation of new data in the thrombosis literature, these recent guidelines once more highlight a key point of our article: clinical decision-making for these complex patients with co-occurrence of venous and arterial thromboembolism requires multidisciplinary collaboration of providers with expertise in the arterial vasculature (eg, neurology, cardiology, and vascular surgery) and those with expertise in the venous vasculature (eg, hematology and pulmonology). Furthermore, system-level programs in anticoagulation stewardship, which are often led by pharmacists with expertise in antithrombotic therapy, are an important tool in providing up-to-date, evidence-based care.87
Acknowledgment
The figures were created with BioRender.com.
Authorship
Contribution: J.E.M. developed the concept and design of the manuscript, wrote the manuscript, and provided final approval; and S.M. contributed to the concept and design, reviewed the manuscript, and gave final approval.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Jori E. May, University of Alabama at Birmingham, 1720 2nd Ave South, NP 2503, Birmingham, AL 35294; email: jemay@uabmc.edu.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal