In hemophilia A dogs, AAV-cFVIII vectors predominantly persist in the liver long-term in nonintegrated episomal forms.
AAV vector integration was seen at low frequencies and occurred commonly in areas of open chromatin, with no effect on gene expression.
Visual Abstract
Gene therapy using adeno-associated virus (AAV) vectors is a promising approach for the treatment of monogenic disorders. Long-term multiyear transgene expression has been demonstrated in animal models and clinical studies. Nevertheless, uncertainties remain concerning the nature of AAV vector persistence and whether there is a potential for genotoxicity. Here, we describe the mechanisms of AAV vector persistence in the liver of a severe hemophilia A dog model (male = 4, hemizygous; and female = 4, homozygous), more than a decade after portal vein delivery. The predominant vector form was nonintegrated episomal structures with levels correlating with long-term transgene expression. Random integration was seen in all samples (median frequency, 9.3e−4 sites per cell), with small numbers of nonrandom common integration sites associated with open chromatin. No full-length integrated vectors were found, supporting predominant episomal vector-mediated long-term transgene expression. Despite integration, this was not associated with oncogene upregulation or histopathological evidence of tumorigenesis. These findings support the long-term safety of this therapeutic modality.
Introduction
Current treatment to prevent bleeding in patients with severe hemophilia A involves regular intravenous infusion of factor VIII (FVIII) concentrates or subcutaneous injection of the bispecific antibody emicizumab (Roche, Basel, Switzerland). The monogenic recessive nature of hemophilia has made it an ideal candidate for a gene therapy approach. The most advanced approach for in vivo gene delivery for hemophilia utilizes adeno-associated virus (AAV) vectors.1 Preclinical studies in large animals, including hemophilia dogs, have described therapeutic transgene expression for over a decade following a single AAV vector infusion.2 Although median follow-up is shorter in clinical studies (hemophilia A: 6 years3,4 and hemophilia B: 6.7 years5), multiple studies are ongoing.1 AAV5-FVIII gene therapy (valoctocogene roxaparvovec, BioMarin, San Rafael, CA) is approved by the US Food and Drug Administration and conditionally approved by the European Medicines Agency for the treatment of adult patients with severe hemophilia A.
The origin of long-term transgene expression from recombinant AAV (rAAV) vectors and potential for genotoxicity remain unclear. In young and selected mouse models, recurrent rAAV integration has been reported at the Rian locus, with development of hepatocellular carcinoma.6,7 In hemophilia dogs, clonal hepatocyte expansion was reported following rAAV treatment, albeit without tumorigenesis.8 Additionally, hepatocellular carcinoma has been reported in an individual with hemophilia B treated with an rAAV vector.9,10 Although vector sequences did not appear to play a pathogenic role in this case, the nature of long-term rAAV vector persistence remains poorly understood. To address this issue, we studied long-term genomic implications of rAAV gene therapy a decade after treatment in the hemophilia dog model. This represents the longest follow-up reported to date post-AAV gene therapy, at the natural lifespan of this animal model. These dogs possess a spontaneous F8 mutation and bleeding phenotype similar to humans with severe hemophilia A.11,12 Here, we describe the frequency, sites of integration, and genomic implications of long-term rAAV vector persistence.
Methods
AAV vector
Canine FVIII (cFVIII) AAV vectors were constructed and administered as described previously.13 Briefly, the AAV-cFVIII expression cassette consisted of a liver-specific transthyretin (TTR) promoter, synthetic intron, noncodon optimized canine B-domain deleted SQ-FVIII cDNA, and synthetic polyadenylation sequence (supplemental Figure 1, available on the Blood website; vector map located in the supplemental Information).2,13 Liver samples (multiple samples from different liver regions) were flash-frozen in liquid nitrogen and stored at −80°C.
Quantification of AAV-cFVIII VG forms
AAV-cFVIII vector genome (VG) extraction and quantification was performed using AllPrep DNA/RNA micro kits (Qiagen, Germantown, MD) and drop-phase droplet digital polymerase chain reaction (ddPCR) assays14 using different primers/probe sets. The cR1-cR11 linked assay measures full-length AAV-cFVIII genomes capable of giving rise to stable cFVIII transcript and the cSQ assay measures overall VGs (full-length and fragments) (supplemental Table 1). DNA was digested with KpnI to separate VG units from concatemers. Plasmid Safe ATP-Dependent DNase (PS-DNase) (Lucigen, Middleton, WI) was used to hydrolyze linear DNA and isolate circular DNA.14 ddPCR of the endogenous gene canine transferrin receptor protein 1 (cTfrc) was performed to provide a normalization reference.
IS analysis by TES and bioinformatic analysis of TES amplicons
Target enrichment sequencing (TES)15 was performed by double-capture using 2 different RNA 120 base pair (bp)-long bait sets, both designed based on 8× tiling (supplemental Figure 2). The first bait set was homologous to the whole vector sequence and the second covered only vector regions diverging between the vector and the dog genome: inverted terminal repeats (ITRs), promoter-transgene junction, transgene exon-exon junctions, and the poly-A. TES was performed first on control samples consisting of vector plasmid spiked into untransduced canine genomic DNA simulating a vector load of 1 VG/cell and nonspiked control. Samples were analyzed in duplicate using 1000 ng DNA per replicate. DNA was sheared to ∼500 bp length using ultrasonicator (Covaris, Woburn, MA) and fragment size was verified by TapeStation (Agilent, Santa Clara, CA). Libraries were prepared using the Agilent SureSelectXT2 kit in line with manufacturer’s instructions. Additional hybridization and PCR enrichment steps were performed on the final library. After library concentration and size distribution evaluation, libraries were sequenced by 2 × 250 bp symmetric paired-end on the Illumina MiSeq platform.
GENE-IS was used for bioinformatic analyses of linear amplification-mediated (LAM)-PCR–derived and TES-derived data.16 Raw sequence data were filtered according to 100% sample barcode identity and sequence quality (Phred 20 for TES and Phred 30 for LAM-PCR). Sequencing reads were aligned to the canine reference genome (canFam3 or canFam4) and vector for IS analysis for both methods.
Vector coverages for each replicate were analyzed and illustrated in Integrative Genome Viewer. The average vector coverage, normalized by the average coverage on the subgenomic regions, was also used to estimate the vector copy number for the analyzed samples. Sequence coverage on the vector and on 5 integration sites (IS) bearing the highest frequencies (≥15%) was analyzed and illustrated in Integrative Genome Viewer. Where applicable, the liftover of IS locations from dog to the human genome (hg38) was carried out using the UCSC liftover tool,17 and their proximity to the cancer genes was assessed using an in-house bioinformatic tool using the cancer gene data available from the Cancer Gene Census database (updated 5 September 2019, v90).18
Statistical analyses
Descriptive data are summarized as mean, median, range, and frequencies. Correlation was performed using Pearson (parametric) or Spearman (nonparametric). Analyses of gene expression were performed using mixed effects models (Dunnett’s adjusted) to account for variance within and between dogs. All tests were two-tailed with a P value <.05 used for statistical significance. Analyses were performed using GraphPad Prism v. 9.0.0 for Windows (San Diego, CA) and SAS software v. 9.4 for modeling the gene expression.
For additional methods please see supplemental Information.
Results
Eight severe hemophilia A dogs were treated with a single infusion of AAV-cFVIII (6e12-2.7e13 vg/kg) at a median of 9.5 months age (Table 1).2 After a median follow-up of 10.8 years (range, 8.2-12.0 years), transgene–derived FVIII:C (chromogenic) activity ranged from 1.8% to 8.6% in 6 responding dogs. Improvement in the bleeding phenotype was seen in all 8 dogs. Analysis of postmortem samples by qPCR/RT-PCR demonstrated the liver as the primary source of vector–derived FVIII expression. There was no evidence of chronic liver disease or liver tumors at postmortem. A full report of the phenotypic outcomes from this study has been previously described.2
Quantification of AAV-cFVIII vectors in the liver following long-term follow-up in the hemophilia A dog model
| ID . | AAV dose vg/kg . | Treatment age (y) . | Follow-up (y) . | CSA FVIII:C (%) . | N . | TES . | LAM-PCR . | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| TES seq . | Total IS seq . | TES unique IS . | Int VCN vg/cell . | LAM-PCR seq . | LAM-PCR unique IS . | LAM-PCR IS per cell . | ||||||
| ALX | AAV6 1.0e13 | 0.7 | 10.5 | 8.6% | 1 2 | 2324084 3525725 | 293 688 | 239 434 | 6.18e−4 1.12e−3 | 87797 n/a | 360 n/a | 6.20e−4 n/a |
| ANG∗ | AAV2 1.5e13 | 1.0 | 11.5 | 0.5% | 1 2 | 2264496 3016959 | 1363 1377 | 310 58 | 8.01e−4 1.50e−4 | 284365 n/a | 167 n/a | 2.88e−4 n/a |
| ELI | AAV2 6.0e12 | 0.6 | 8.2 | † | 1 2 | 2915252 2406745 | 385 656 | 269 496 | 6.95e−4 1.28e−3 | 257439 n/a | 213 n/a | 3.67e−4 n/a |
| FLO | AAV8 1.0e13 | 1.0 | 10.5 | 1.8% | 1 2 | 3057875 3237835 | 838 887 | 362 413 | 9.35e−4 1.07e−3 | n/a n/a | n/a n/a | n/a n/a |
| JUN | AAV2 2.7e13 | 1.0 | 12.0 | 7.2% | 1 2 | 2286381 2494473 | 948 700 | 550 534 | 1.42e−3 1.38e−3 | 160937 n/a | 380 n/a | 6.55e−4 n/a |
| MG∗ | AAV6 1.7e13 | 0.5 | 11.5 | 0.3% | 1 2 | 2597851 2525320 | 436 152 | 49 105 | 1.27e−4 2.71e−4 | 146726 n/a | 67 n/a | 1.15e−4 n/a |
| MZ | AAV6 1.0e13 | 1.3 | 10.1 | 7.9% | 1 2 | 2369218 2355983 | 650 458 | 243 353 | 6.28e−4 9.12e−4 | n/a n/a | n/a n/a | n/a n/a |
| VC | AAV2 1.5e13 | 0.7 | 11.0 | 2.9% | 1 2 | 3855884 2012102 | 1349 353 | 1055 276 | 2.73e−3 7.13e−4 | n/a 228238 | n/a 196 | n/a 3.38e−4 |
| TB | Control | n/a | n/a | n/a | 1 | 2805661 | 4 | 4 | 1.03e−5 | n/a | n/a | n/a |
| ID . | AAV dose vg/kg . | Treatment age (y) . | Follow-up (y) . | CSA FVIII:C (%) . | N . | TES . | LAM-PCR . | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| TES seq . | Total IS seq . | TES unique IS . | Int VCN vg/cell . | LAM-PCR seq . | LAM-PCR unique IS . | LAM-PCR IS per cell . | ||||||
| ALX | AAV6 1.0e13 | 0.7 | 10.5 | 8.6% | 1 2 | 2324084 3525725 | 293 688 | 239 434 | 6.18e−4 1.12e−3 | 87797 n/a | 360 n/a | 6.20e−4 n/a |
| ANG∗ | AAV2 1.5e13 | 1.0 | 11.5 | 0.5% | 1 2 | 2264496 3016959 | 1363 1377 | 310 58 | 8.01e−4 1.50e−4 | 284365 n/a | 167 n/a | 2.88e−4 n/a |
| ELI | AAV2 6.0e12 | 0.6 | 8.2 | † | 1 2 | 2915252 2406745 | 385 656 | 269 496 | 6.95e−4 1.28e−3 | 257439 n/a | 213 n/a | 3.67e−4 n/a |
| FLO | AAV8 1.0e13 | 1.0 | 10.5 | 1.8% | 1 2 | 3057875 3237835 | 838 887 | 362 413 | 9.35e−4 1.07e−3 | n/a n/a | n/a n/a | n/a n/a |
| JUN | AAV2 2.7e13 | 1.0 | 12.0 | 7.2% | 1 2 | 2286381 2494473 | 948 700 | 550 534 | 1.42e−3 1.38e−3 | 160937 n/a | 380 n/a | 6.55e−4 n/a |
| MG∗ | AAV6 1.7e13 | 0.5 | 11.5 | 0.3% | 1 2 | 2597851 2525320 | 436 152 | 49 105 | 1.27e−4 2.71e−4 | 146726 n/a | 67 n/a | 1.15e−4 n/a |
| MZ | AAV6 1.0e13 | 1.3 | 10.1 | 7.9% | 1 2 | 2369218 2355983 | 650 458 | 243 353 | 6.28e−4 9.12e−4 | n/a n/a | n/a n/a | n/a n/a |
| VC | AAV2 1.5e13 | 0.7 | 11.0 | 2.9% | 1 2 | 3855884 2012102 | 1349 353 | 1055 276 | 2.73e−3 7.13e−4 | n/a 228238 | n/a 196 | n/a 3.38e−4 |
| TB | Control | n/a | n/a | n/a | 1 | 2805661 | 4 | 4 | 1.03e−5 | n/a | n/a | n/a |
Control, untreated hemophilia A control dog; CSA, chromogenic substrate FVIII activity assay, using a pooled normal canine standard (12-20 normal dogs); Epi VCN, episomal vector copy number; Int VCN, integrated vector copy number; N, liver sample number (ie, ALX sample 1 = ALX-1); n/a, not available; Seq, sequencing reads.
Nonresponder
Terminal sample not available: earlier CSA FVIII:C on-study: 3.4% to 5.7%.
As an extension to these studies, the regional distribution of AAV-cFVIII VG copies and cFVIII messenger RNA (mRNA) expression was evaluated using DNA and mRNA coisolated from multiple liver samples by ddPCR. In the majority of AAV-cFVIII–treated dogs, little intra-animal regional variation in hepatic vector copy number or cFVIII mRNA expression was seen, whereas in some, more marked regional variation was present (eg, JUN) (Figure 1).
Regional variation of AAV-cFVIII seen in the liver after long-term follow-up in the hemophilia A dog model, quantified using drop-phase ddPCR. NR, dogs with plasma FVIII:C levels that remained below the limit of detection but showed evidence of improved whole blood clot times compared with untreated hemophilia A dogs.2 (A) AAV-cFVIII VG copies quantified in copies per diploid genome. (B) cFVIII mRNA expression normalized to beta-2-microglobulin (B2M). NR, nonresponder; vgDNA, vector genome DNA.
Regional variation of AAV-cFVIII seen in the liver after long-term follow-up in the hemophilia A dog model, quantified using drop-phase ddPCR. NR, dogs with plasma FVIII:C levels that remained below the limit of detection but showed evidence of improved whole blood clot times compared with untreated hemophilia A dogs.2 (A) AAV-cFVIII VG copies quantified in copies per diploid genome. (B) cFVIII mRNA expression normalized to beta-2-microglobulin (B2M). NR, nonresponder; vgDNA, vector genome DNA.
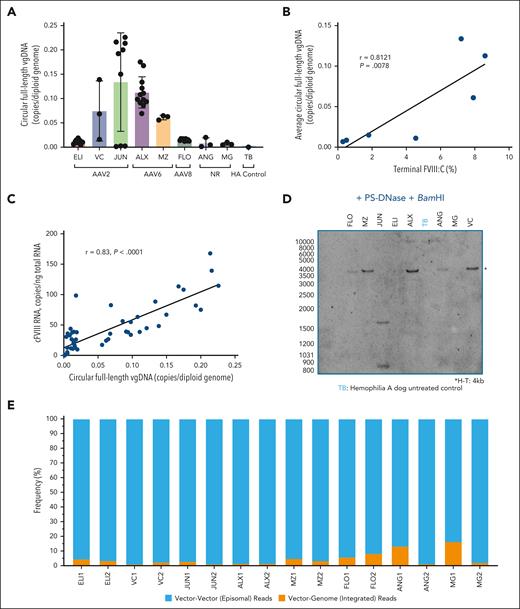
Preclinical and clinical studies of AAV5-hFVIII-SQ transduced livers have demonstrated that circularized monomeric and concatemeric episomes are the major AAV DNA forms associated with long-term transgene expression.14,19 Here, we evaluated the VG forms mediating long-term expression (ie, episomal or integrated) using 3 orthogonal methodologies. Firstly, liver circular episomal AAV-cFVIII was enriched by digestion with PS-DNase and followed by KpnI to allow enumeration of transgene cassettes in the circular genomes.20 Absolute quantification of full-length vector units was performed using drop-phase ddPCR with primers/probes to the proximal end of the 5′ and 3′ ITRs (D-segment).19 Full-length circular episomes were detected using ddPCR in the liver of all AAV-cFVIII–treated dogs (Figure 2A). Importantly, this assay underestimates the number of circular VGs due to shearing/linearization of large concatemeric episomes during DNA extraction, and degradation of 25% to 35% of episomes by PS-DNase.19,20 A significant positive correlation was observed between the circular full-length episomal vector levels and terminal FVIII:C (r = 0.812; P = .0078; Figure 2B) and cFVIII RNA (r = 0.83; P < .0001; Figure 2C), suggesting FVIII expression was primarily derived from episomal vectors. These findings were confirmed using Southern blotting (Figure 2D), performed on a single sample from each dog, demonstrating primarily head-to-tail configured full-length episomes with band intensities in each sample correlating with circular genome copies measured by ddPCR (supplemental Figure 3).
Episomal AAV-cFVIII vector forms detected in the liver after long-term follow-up, with correlation between vector copies and FVIII expression. (A) Circular full-length AAV-cFVIII VGs, detected by drop-phase ddPCR following treatment with PS-DNase and KpnI to enrich circular genomes and quantify full-length monomers. (B) Strong correlation between circular full-length VG copies and terminal FVIII:C levels measured by chromogenic substrate assay. For ELI, where terminal FVIII:C was unavailable, a mean of earlier FVIII:C on-study was used. (C) Strong correlation (r = 0.83; P < .0001) between full-length episomal VG copies and cFVIII mRNA expression, supporting episomal AAV-cFVIII as the primary source of FVIII expression. (D) Southern blotting, following PS-DNase and BamHI, demonstrates full-length AAV-cFVIII VGs in an H-T configuration (band size 4 kb). Sample from JUN contains 2 fragments (∼1600 and 850 bp) suggesting AAV-cFVIII truncation. (E) TES comparing frequencies of V-V, possibly episomal (blue) to vector-canine genome V-G, integrated (orange) sequencing reads demonstrating the majority resulted from V-V (episomal) reads.
Episomal AAV-cFVIII vector forms detected in the liver after long-term follow-up, with correlation between vector copies and FVIII expression. (A) Circular full-length AAV-cFVIII VGs, detected by drop-phase ddPCR following treatment with PS-DNase and KpnI to enrich circular genomes and quantify full-length monomers. (B) Strong correlation between circular full-length VG copies and terminal FVIII:C levels measured by chromogenic substrate assay. For ELI, where terminal FVIII:C was unavailable, a mean of earlier FVIII:C on-study was used. (C) Strong correlation (r = 0.83; P < .0001) between full-length episomal VG copies and cFVIII mRNA expression, supporting episomal AAV-cFVIII as the primary source of FVIII expression. (D) Southern blotting, following PS-DNase and BamHI, demonstrates full-length AAV-cFVIII VGs in an H-T configuration (band size 4 kb). Sample from JUN contains 2 fragments (∼1600 and 850 bp) suggesting AAV-cFVIII truncation. (E) TES comparing frequencies of V-V, possibly episomal (blue) to vector-canine genome V-G, integrated (orange) sequencing reads demonstrating the majority resulted from V-V (episomal) reads.
Complementary analysis was performed using targeted enrichment next-generation sequencing (TES, Figure 2E) (2 samples per dog). Sequencing reads containing vector-vector (V-V) or vector-genome (V-G) junctions were analyzed providing estimates of the proportion of episomal and integrated forms. The majority (95.4%; range, 83.6%-98.9%) of sequencing reads resulted from V-V (approximated as episomal) reads, with only 4.6% (range, 1.2%-16.4%) resulting from V-G (integrated) reads (Table 1; Figure 2E). Previous in vitro studies of wild-type AAV have suggested that integrated vector concatemers are infrequent.21 Collectively, results obtained from orthogonal methods support that AAV predominantly persists within the liver in an episomal form a decade after vector delivery. However, we cannot rule out the presence of integrated concatemers, as was recently reported using long-read sequencing in nonhuman primates treated with AAV8 vectors encoding rhesus low-density lipoprotein receptor, human low-density lipoprotein receptor, or green fluorescent protein.22
Low frequencies of integrations were seen predominantly in intergenic regions
Although AAV-cFVIII vectors predominantly persist episomally, integration was seen in all liver samples analyzed. Integration frequencies were calculated using TES and confirmed using an orthogonal LAM-PCR approach (Table 1; supplemental Figure 2). Using TES, there were 43 246 183 sorted sequencing reads, with 11 533 IS reads. This resulted in 5746 uniquely mappable IS and a mean integration frequency of 9.3e−4 IS per cell (range, 1.21e−4 to 2.72e−3), equating to 0.93 integration events per 1000 cells (Table 1). Integration frequency correlated (r = 0.94; P = .0005) with hepatic VG copies. Characterization of IS locations relative to annotated genes, demonstrated the majority (93.8%) occurred in intergenic regions of the canine genome (Figure 3A). An overview of unique in-gene insertions (total = 355, of which F8 = 195) is provided in Table 2 and supplemental Table 3.
TES evaluation of IS. (A) Location of unique IS relative to annotated canine genes (canFam3) detected using TES. The majority (93.8%) of unique IS were located in intergenic regions. For in-gene insertions, 206 of 355 (58%) fell within an intron and 149 of 355 (42%) in an exon. Upstream insertions are defined as intergenic regions 5′ of the closest gene, and downstream insertions are 3′ of the closest gene. In-gene insertions are defined as those located within the transcriptional unit. (B-D) Cumulative sequence counts of the top-10 IS were identified. (B) Most (10/16) samples exhibited integration profiles in which the top IS demonstrated frequencies <10% of the total sequences. Representative examples include VC-2 and FLO-1. (C) 4 of 16 samples had at least 1 IS with a frequency of >10% and <30% of the total IS sequence count. Representative examples include JU-1 and MZ-1. (D) 2 of 16 samples had at least 1 IS with a frequency of >30%. Both of these samples were obtained from nonresponding dogs (ANG-2 and MG-1). Refer to supplemental Figure 4 for top-10 IS data for all 16 samples analyzed. NR, nonresponder.
TES evaluation of IS. (A) Location of unique IS relative to annotated canine genes (canFam3) detected using TES. The majority (93.8%) of unique IS were located in intergenic regions. For in-gene insertions, 206 of 355 (58%) fell within an intron and 149 of 355 (42%) in an exon. Upstream insertions are defined as intergenic regions 5′ of the closest gene, and downstream insertions are 3′ of the closest gene. In-gene insertions are defined as those located within the transcriptional unit. (B-D) Cumulative sequence counts of the top-10 IS were identified. (B) Most (10/16) samples exhibited integration profiles in which the top IS demonstrated frequencies <10% of the total sequences. Representative examples include VC-2 and FLO-1. (C) 4 of 16 samples had at least 1 IS with a frequency of >10% and <30% of the total IS sequence count. Representative examples include JU-1 and MZ-1. (D) 2 of 16 samples had at least 1 IS with a frequency of >30%. Both of these samples were obtained from nonresponding dogs (ANG-2 and MG-1). Refer to supplemental Figure 4 for top-10 IS data for all 16 samples analyzed. NR, nonresponder.
In-gene IS mapped to the canine F8 gene using TES
| ID . | Sample . | Total IS . | F8 intron IS . | F8 exon IS . | True F8 IS (%) . | Undetermined F8 IS (%) . |
|---|---|---|---|---|---|---|
| ANG | 1 | 4 | 2 | 2 | 50.0 | 50.0 |
| ANG | 2 | 113 | 0 | 113 | 0 | 100.0 |
| ELI | 1 | 11 | 9 | 2 | 81.8 | 18.2 |
| ELI | 2 | 13 | 1 | 12 | 7.7 | 92.3 |
| VC | 1 | 63 | 22 | 41 | 34.9 | 65.1 |
| VEC | 2 | 12 | 5 | 7 | 41.7 | 58.3 |
| ALX | 1 | 5 | 1 | 4 | 20.0 | 80.0 |
| ALX | 2 | 10 | 2 | 8 | 20.0 | 80.0 |
| JUN | 1 | 18 | 6 | 12 | 33.3 | 66.6 |
| JUN | 2 | 42 | 9 | 33 | 21.4 | 78.6 |
| MZ | 1 | 5 | 2 | 3 | 40.0 | 60.0 |
| MZ | 2 | 12 | 6 | 6 | 50.0 | 50.0 |
| FLO | 1 | 13 | 9 | 4 | 69.2 | 30.8 |
| FLO | 2 | 26 | 21 | 5 | 80.8 | 19.2 |
| MG | 1 | 5 | 2 | 3 | 40.0 | 60.0 |
| MG | 2 | 0 | 0 | 0 | 0.0 | 0.0 |
| ID . | Sample . | Total IS . | F8 intron IS . | F8 exon IS . | True F8 IS (%) . | Undetermined F8 IS (%) . |
|---|---|---|---|---|---|---|
| ANG | 1 | 4 | 2 | 2 | 50.0 | 50.0 |
| ANG | 2 | 113 | 0 | 113 | 0 | 100.0 |
| ELI | 1 | 11 | 9 | 2 | 81.8 | 18.2 |
| ELI | 2 | 13 | 1 | 12 | 7.7 | 92.3 |
| VC | 1 | 63 | 22 | 41 | 34.9 | 65.1 |
| VEC | 2 | 12 | 5 | 7 | 41.7 | 58.3 |
| ALX | 1 | 5 | 1 | 4 | 20.0 | 80.0 |
| ALX | 2 | 10 | 2 | 8 | 20.0 | 80.0 |
| JUN | 1 | 18 | 6 | 12 | 33.3 | 66.6 |
| JUN | 2 | 42 | 9 | 33 | 21.4 | 78.6 |
| MZ | 1 | 5 | 2 | 3 | 40.0 | 60.0 |
| MZ | 2 | 12 | 6 | 6 | 50.0 | 50.0 |
| FLO | 1 | 13 | 9 | 4 | 69.2 | 30.8 |
| FLO | 2 | 26 | 21 | 5 | 80.8 | 19.2 |
| MG | 1 | 5 | 2 | 3 | 40.0 | 60.0 |
| MG | 2 | 0 | 0 | 0 | 0.0 | 0.0 |
IS mapped within a F8 intron were deemed as “true” IS as these regions are not represented in the AAV-cFVIII vector. IS mapped to a F8 exon were considered “undetermined,” as these could represent either true exonic insertions or V-V insertion events. Overall, 97/352 (27.6%) insertion sites were located in F8 introns.
Evaluation of IS abundance
Individual sample IS frequencies were determined and the 10 most abundant (top-10 IS) are shown in Figure 3B-D and supplemental Figure 4. Differences in IS profiles were seen between dogs and samples. Clonal abundance was evaluated from frequencies of unique IS in individual samples. This provides approximation of abundance, with thresholds based on data from retroviral integration studies.23 Most samples (10/16) exhibited integration profiles in which each of the top-10 IS had a frequency of <10% of the total IS sequence count (average = 1.59%) (Figure 3B). For the 6 samples with IS frequencies >10%, the average frequency for the top-10 IS was 6.03% (Figure 3C). IS with frequencies >30% were only seen in single samples in the 2 nonresponding dogs (MG and ANG) (Figure 3D) with plasma FVIII:C below the limit of detection but improved whole blood clot times post-AAV-cFVIII as previously described.2 MG-1 had 2 IS ∼162 kb upstream of CCND1 (33.0% and 53.0%) and ANG-2 had 1 IS ∼424 kb upstream of MIR320 (31.7%). In these 2 samples, the top-10 IS accounted for >90% of the total IS, although this is skewed in MG-1 by the low total number of IS. Both MG and ANG had low levels of total AAV-cFVIII vector genome DNA (vgDNA) (Figure 1A) and circular full-length vgDNA (Figure 2A) suggesting that low rates of vector integration may account for the increased IS frequency observed. Data from the orthogonal LAM-PCR strategy is provided in supplemental Figure 5. In summary, these datasets demonstrate the presence of specific IS clones at higher abundances in 2 samples when compared with the overall polyclonal integration profile observed in the majority of samples.
No deletions seen in the host genome at IS
We then evaluated the implications of AAV-cFVIII integration on adjacent host genome regions for the higher frequency (>15%) IS: MIR320 (ANG-2, n = 2), MIR1296 (JUN-1, n = 2), KCNIP2 (ALX-2), MET (MZ-1), and CCND1 (MG-1) (supplemental Table 4). Using TES short-read sequencing, possible deletions were seen for MIR320 (ANG-2: second IS 3 bp deletion), MIR1296 (JUN-1: second IS 176 bp deletion), MET (MZ-1: 185 bp deletion), and CCND1 (MG-1: 70 bp deletion) (supplemental Figure 6). Long-read nanopore sequencing was used to further investigate these putative deletions. After sequence read alignment, no deletions were seen, which likely represent short-read sequencing artifacts using TES. In this small sample of IS, these data suggest that integration occurred without significant impact on the surrounding host genome.
CISs were seen with no effect on gene expression
Analysis of the distribution of IS demonstrated these occurred mostly randomly throughout the canine genome (Figure 4A). A nonbiased systems biology approach was then used to evaluate whether IS formed into clusters or common IS (CIS). Using the TES and LAM-PCR methodologies, 37 and 4 CIS with a CIS order ≥5 were seen, respectively (Table 3; supplemental Table 5). The most frequent CIS are shown in Figure 4B, on chromosome 14 (ABCB1), 28 (KCNIP2), and X (F8 and CLIC2). The CIS predominantly occurred in intergenic regions, with the only common in-gene CIS, occurring within the F8 and ALB loci. No differences were observed in the hepatic gene expression of ABCB1, CLIC2, KCNIP2, and ALB in AAV-cFVIII–treated, compared with that of normal or untreated hemophilia dogs (Figure 4C-E; supplemental Figure 7; supplemental Table 6). Of note, samples used were not coisolated from those used for TES and LAM-PCR, and multiple samples were analyzed per dog to ensure accurate representation. Importantly, none of these CIS are located within genomic regions linked with wild-type AAV integration or regions associated with adverse events (clonal outgrowth or malignant transformation) in clinical gene therapy trials.23-30 No evidence of tumorigenesis was observed on histopathological assessment (supplemental Table 7).2
Features and cellular implication sites of hepatic AAV-cFVIII integration after long-term follow-up in the hemophilia A dog model. (A) Heat map of IS in 10 MB window sizes, demonstrating integration across the canine genome detected by TES; frequencies displayed on a density scale ranging from 0 IS/10 Mb to >697 IS/10 Mb, with highest frequency IS shown in yellow and red. (B) Representation of CIS seen within 10 Mb window sizes on chromosomes 14 (ABCB1), 28 (KCNIP2), and X (F8 and CLIC2). (C-E) No significant dysregulation in gene expression of genes in proximity to CIS in the liver of AAV-cFVIII treated hemophilia A dogs, compared with untreated hemophilia A or normal dogs; quantification by drop-phase ddPCR and normalized to B2M expression. (F) No dysregulation in MET expression in the liver of AAV-cFVIII treated hemophilia A dogs, compared with untreated hemophilia A or normal dogs; quantification by ddPCR and normalized to B2M expression. (G) Comparison of IS frequencies in proximity (≤100 kb) to cancer genes to simulated data sets (n = 10 000), demonstrated this frequency is within the predicted normal range (0.065-0.084). (H) Evaluation of association (P < .05) of IS with areas of chromatin accessibility (ATAC-seq) and methylation status (bisulfite sequencing). N-dog, normal dog; NTC, no template control.
Features and cellular implication sites of hepatic AAV-cFVIII integration after long-term follow-up in the hemophilia A dog model. (A) Heat map of IS in 10 MB window sizes, demonstrating integration across the canine genome detected by TES; frequencies displayed on a density scale ranging from 0 IS/10 Mb to >697 IS/10 Mb, with highest frequency IS shown in yellow and red. (B) Representation of CIS seen within 10 Mb window sizes on chromosomes 14 (ABCB1), 28 (KCNIP2), and X (F8 and CLIC2). (C-E) No significant dysregulation in gene expression of genes in proximity to CIS in the liver of AAV-cFVIII treated hemophilia A dogs, compared with untreated hemophilia A or normal dogs; quantification by drop-phase ddPCR and normalized to B2M expression. (F) No dysregulation in MET expression in the liver of AAV-cFVIII treated hemophilia A dogs, compared with untreated hemophilia A or normal dogs; quantification by ddPCR and normalized to B2M expression. (G) Comparison of IS frequencies in proximity (≤100 kb) to cancer genes to simulated data sets (n = 10 000), demonstrated this frequency is within the predicted normal range (0.065-0.084). (H) Evaluation of association (P < .05) of IS with areas of chromatin accessibility (ATAC-seq) and methylation status (bisulfite sequencing). N-dog, normal dog; NTC, no template control.
Location of the top 10 CIS detected by TES and LAM-PCR
| . | Rank . | Events . | Chr . | Mean integration locus . | Dimension (bp) . | Gene . | Entropy . |
|---|---|---|---|---|---|---|---|
| TES | 1∗ | 740 | 28 | 14066254 | 30 324 | KCNIP2 | 0.90 |
| 2∗ | 521 | X | 123771584 | 25 052 | CLIC2 | 0.94 | |
| 3∗ | 373 | 14 | 16288512 | 37 578 | ABCB1 | 0.94 | |
| 4∗ | 361 | X | 122970340 | 146 353 | F8 | 0.90 | |
| 5 | 69 | 5 | 75600480 | 17 415 | LOC479649 | 0.88 | |
| 6 | 30 | 13 | 62161330 | 16 400 | ALB | 0.90 | |
| 7 | 26 | 4 | 14746173 | 72 601 | MIR1296 | 0.93 | |
| 8 | 25 | 1 | 121363006 | 948 | MIR578 | 0.97 | |
| 9 | 18 | 13 | 32788006 | 754 | MIR30D | 0.99 | |
| 10 | 15 | 6 | 43486296 | 3 975 | MIR8880 | 0.67 | |
| LAM-PCR | 1∗ | 45 | 28 | 14062005 | 11 015 | KCNIP2 | 0.87 |
| 2∗ | 29 | X | 123771226 | 16 893 | CLIC2 | 0.93 | |
| 3∗ | 11 | 14 | 16281704 | 7 201 | ABCB1 | 0.99 | |
| 4∗ | 10 | X | 122948039 | 80 519 | F8 | 0.88 |
| . | Rank . | Events . | Chr . | Mean integration locus . | Dimension (bp) . | Gene . | Entropy . |
|---|---|---|---|---|---|---|---|
| TES | 1∗ | 740 | 28 | 14066254 | 30 324 | KCNIP2 | 0.90 |
| 2∗ | 521 | X | 123771584 | 25 052 | CLIC2 | 0.94 | |
| 3∗ | 373 | 14 | 16288512 | 37 578 | ABCB1 | 0.94 | |
| 4∗ | 361 | X | 122970340 | 146 353 | F8 | 0.90 | |
| 5 | 69 | 5 | 75600480 | 17 415 | LOC479649 | 0.88 | |
| 6 | 30 | 13 | 62161330 | 16 400 | ALB | 0.90 | |
| 7 | 26 | 4 | 14746173 | 72 601 | MIR1296 | 0.93 | |
| 8 | 25 | 1 | 121363006 | 948 | MIR578 | 0.97 | |
| 9 | 18 | 13 | 32788006 | 754 | MIR30D | 0.99 | |
| 10 | 15 | 6 | 43486296 | 3 975 | MIR8880 | 0.67 | |
| LAM-PCR | 1∗ | 45 | 28 | 14062005 | 11 015 | KCNIP2 | 0.87 |
| 2∗ | 29 | X | 123771226 | 16 893 | CLIC2 | 0.93 | |
| 3∗ | 11 | 14 | 16281704 | 7 201 | ABCB1 | 0.99 | |
| 4∗ | 10 | X | 122948039 | 80 519 | F8 | 0.88 |
Chr, chromosome; entropy, contribution of CIS within individual or different samples, this ranges from 0 to 1, reflecting homogeneous (ie, low values found in a single sample) to heterogeneous (ie, high values found in several samples) CIS. High entropy values within this study signify the heterogeneous nature of the CIS.
CIS seen in both sequencing methodologies.
No enrichment of integration seen relative to known human cancer genes
We then evaluated whether IS were enriched in proximity to known oncogenes. Because there is no canine cancer gene database, a liftover of IS referenced to canFam4 was performed to the human genome (hg38). Successfully lifted over IS (3538/5755) coordinates were referenced against 7 human cancer gene databases (supplemental Table 8) for locations proximal (≤100 kb) to the transcriptional start site of known oncogenes. Using the curated Sanger database,18,31 8.4% (n = 297) IS occurred <100 kb from a known cancer gene. This included 13 of the top-10 IS, proximal to MET = 5, MTCP1 = 4, NCOA4 = 1, LCP1 = 1, MALAT1 = 1, and NF2 = 1 (supplemental Table 9); all with relative frequencies <20%. The highest IS frequency (18.2%) was seen in MZ-1, proximal to the hepatocyte growth factor receptor (MET) locus, however, no change in hepatic MET expression was seen in liver samples from any of the AAV-cFVIII–treated dogs compared with normal or untreated hemophilia dogs (Figure 4F). IS frequencies relative to cancer genes were then compared with 10 000 simulated data sets. This demonstrated IS frequencies fell within the predicted normal range (0.065-0.084), indicating no enrichment in IS proximal to human cancer genes (Figure 4G).
CIS occurred at chromatin accessible regions of the canine genome
We then went on to evaluate genomic mechanisms underlying these CIS. Firstly, top-10 TES CIS (canFam3) were analyzed to determine whether there was sequence homology between IS regions and vector sequences. Excluding CIS within the F8 gene, no homology was seen comparing CIS sequences with vector sequences. In view of homology between the cFVIII vector and the native canine F8 gene, we analyzed IS mapped to the F8 locus. F8 intronic IS were regarded as true IS due to lack of these elements within the vector, and F8 exonic IS were considered as either true integration events or an internal V-V junction sequence. Although some F8 IS could not be excluded from being false positives due to exonic location, integration was seen within introns of the native canine F8 gene (Table 2). We then evaluated whether integration occurred more frequently in regions of increased chromatin accessibility in the canine genome (canFam4) using ATAC-seq (transposase-accessible chromatin sequencing). Analyses of regions surrounding IS sites were performed on dog liver samples (n = 7, Figure 4H) and associations of ATAC-seq scores were calculated using receiver operating characteristic (ROC) scores compared with a random sample. ROC scores varied from 0.4 (significant negative association) to 0.6 (significant positive association).32 Although, for regions >10 kbp, we did not observe many deviations in ROC score from 0.5, increased ROC scores were seen for IS regions of 1 kbp down to 50 bp. This supports the hypothesis that focused regional enhanced chromatin accessibility has a facilitatory influence on vector integration. To determine whether DNA methylation plays a role in AAV integration, IS distributions were compared with whole genome bisulfite sequencing (Methyl-seq) results (3 canine datasets, 47 000 000 sites) and the average measurement of the overall enrichment for corresponding genomic regions determined by ATAC-seq. No association with ROC values for methylation were seen. These results suggest an association between integration and regions of higher chromatin accessibility, which could enable passive vector integration at locations where DNA is loosened from the nucleosome complex.
No full-length integrated rAAV vectors were detected
Finally, we evaluated whether full-length integrated AAV-cFVIII could be detected, which could result in transgene expression. TES sequencing reads were analyzed to determine whether these were homogeneously represented and/or if recurrent deletions had occurred. Most samples (15/16) demonstrated homogeneous coverage (supplemental Figures 8 and 9). One sample (ANG-2) displayed high coverage in the promoter and poly(A), with no transgene reads, indicating partially deleted VGs. Irregular coverage profiles were seen for 2 other samples (ANG-2 and MG-1), with peaks downstream of the 5′ ITR, promoter, and at exon-exon junctions but without interruptions that could imply vector breakage. Although this approach provides an impression of overall vector sequence coverage, it cannot provide information on genome contiguity.
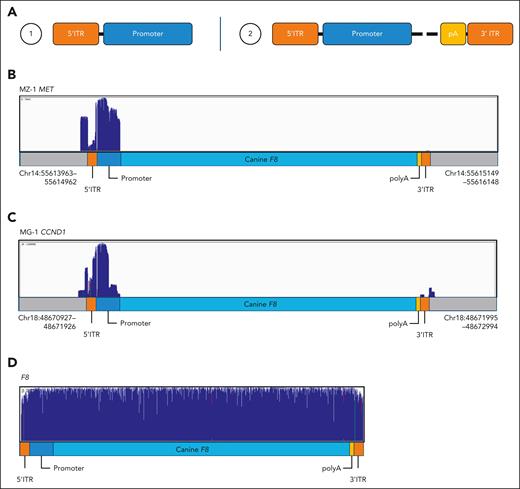
To address this, long-read sequencing was performed in 8 higher (>10%) frequency IS (supplemental Table 10). Sorted reads were aligned to their reference IS and demonstrated that no full-length integrated vector copies were found (Figure 5; supplemental Figure 10). For the IS investigated, 6 had structures comprising the 5′ ITR and most of the promoter and 3 showed parts of the poly-A and 3′ ITR (Figure 5A). For most (7 out of 8), the 5′ ITR and parts of the promoter were retrieved (Figure 5B), and in 3 samples, parts of the poly(A) and 3′ ITR were also retrieved (Figure 5C). Examples of sequencing reads from different size classes (0-500 bp, 501-1000 bp, and >1000 bp) were investigated to confirm these identified structures. For most samples, extracted longer reads confirmed the integrated structure shown by the coverage plots. In particular, several vector fragments were retrieved suggesting vector recombination within integrated forms. However, this approach is not able to distinguish between truly rearranged forms and chimeric reads arising from the library preparation. Finally, to exclude sequencing bias from the ITRs and vector sequences that might limit full-length sequence detection, a cF8x104 plasmid control (AAV_TTR_FVIII) was amplified and sequenced using the same protocol (Figure 5D; supplemental Figure 11). This demonstrated uniform coverage of the full-length plasmid control, excluding the possibility that full-length integrated vectors could not be retrieved. These findings further support the observation that full vector sequences predominantly (and possibly exclusively) persist in episomal rather than as integrated forms.
Evaluation of vector integrity by long-read sequencing. Long range sequencing was performed for 8 IS with a higher (>10%) observed frequency. (A) Suggested structures of integrated AAV-cFVIII vector for selected IS. Structure 1 included the 5′ ITR and part of the promoter (identified in ANG-2 MIR320, MZ-1 MET, ANG-1 MIR1296 and ANG-2 MET). Structure 2 included the 5′ ITR, part of the promoter, parts of the poly-A and 3′ ITR (identified in MG-1 CCND1, MZ-1 CCND1 and JU-1 MIR1296). (B) Vector coverage for samples MZ-1 MET. Sequencing data aligned to putative IS reference, including the AAV_TTR_FVIII vector at the IS location retrieved by TES. Scale is set to 0 to 70 664 reads. (C) Vector coverage for sample MG-1 CCND1. Sequencing data aligned to putative IS reference, including the AAV-cFVIII vector at the IS location retrieved by TES. Scale is set to 0 to 120 555 reads. (D) Coverage for all sorted reads obtained from the AAV-cFVIII amplicon. Sequencing data aligned to the AAV-cFVIII vector sequence. Scale is set to 0 to 3 131 072 reads. For other examples see supplemental Figure 10.
Evaluation of vector integrity by long-read sequencing. Long range sequencing was performed for 8 IS with a higher (>10%) observed frequency. (A) Suggested structures of integrated AAV-cFVIII vector for selected IS. Structure 1 included the 5′ ITR and part of the promoter (identified in ANG-2 MIR320, MZ-1 MET, ANG-1 MIR1296 and ANG-2 MET). Structure 2 included the 5′ ITR, part of the promoter, parts of the poly-A and 3′ ITR (identified in MG-1 CCND1, MZ-1 CCND1 and JU-1 MIR1296). (B) Vector coverage for samples MZ-1 MET. Sequencing data aligned to putative IS reference, including the AAV_TTR_FVIII vector at the IS location retrieved by TES. Scale is set to 0 to 70 664 reads. (C) Vector coverage for sample MG-1 CCND1. Sequencing data aligned to putative IS reference, including the AAV-cFVIII vector at the IS location retrieved by TES. Scale is set to 0 to 120 555 reads. (D) Coverage for all sorted reads obtained from the AAV-cFVIII amplicon. Sequencing data aligned to the AAV-cFVIII vector sequence. Scale is set to 0 to 3 131 072 reads. For other examples see supplemental Figure 10.
Discussion
This study has provided detailed analyses of the fate and forms of vectors over a decade post-rAAV-cFVIII treatment. Using orthogonal strategies, we demonstrated that the majority of vectors exist long-term in episomal structures that correlate with transgene RNA expression. These findings are in keeping with descriptions of episomal forms seen 2 to 4 years following AAV5-hFVIII-SQ administration in humans.14 Integration frequencies are similar to those reported after shorter follow-up for an AAV2/5-cohPBGD vector used for treatment of acute intermittent porphyria in nonhuman primates (4 weeks, 2.0e−4 IS per cell) and humans (52 weeks, 1.17e−3 IS per cell) suggesting integration events occur early after gene transfer and remain stable over time.33 These integration frequencies are lower than seen with lentiviral vectors and the spontaneous somatic mutation rate.34 Analyzing integrated vector sequences, we only found fragmented and rearranged copies, which is similar to the findings from other investigators.8 Collectively, this provides evidence that long-term FVIII expression was episomally derived, although we acknowledge further study is required on the source of transgene expression (episomal or integrated) based on a recent study reporting the presence of integrated AAV concatemers in nonhuman primates.22
Although integration occurred throughout the canine genome, we identified CIS. Using 2 different sequencing strategies, these were identified in proximity to 5 genes: KCNIP2, CLIC2, ABCB1, F8, and ALB, all of which are liver-expressed. Recurrent rAAV IS have been described in murine and canine studies. In mice, the predominant site of recurrent integration is within the Rian locus, with the majority occurring in the mir341 locus, which is not encoded within the canine or human genomes.35 In a similar hemophilia A dog study, recurrent IS were seen in proximity to EGR2, EGR3, CCND1, ALB, and DUSP1.8 An overlap is seen in the genomic loci of CIS in both dog studies, with the exception of DUSP1 (Table 4).8 Considering that IS are assigned to the closest annotated gene, the different canine genome annotation tracks used in this and the previous study may account for these differences. Nonetheless, we did not observe evidence of dysregulated expression of adjacent genes for the CIS seen in AAV-cFVIII treated dogs, compared with normal or untreated hemophilia dogs (supplemental Table 11). With albumin being highly expressed in hepatocytes, this locus has an accessible chromatin landscape facilitating vector integration. This is supported by our findings of high ATAC-seq scores in CIS regions, suggesting that integration occurs through “passive invasion” of the genome at regions of chromatin accessibility.
Overlapping integration clusters in 2 canine AAV-cFVIII studies after long-term follow-up
| Chr . | UNC integration clusters . | Queen’s TES CIS . | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Hits . | Start . | End . | Width . | Gene∗ . | CIS (no. of events) . | Mean locus . | Width . | Gene∗ . | |
| 4 | 7 | 14530145 | 14924592 | 39 448 | EGR2 | 7 (26) | 14746173 | 72 601 | MIR1296 |
| 4 | 26 | 39589684 | 39589877 | 194 | DUSP1 | nr | nr | nr | nr |
| 13 | 5 | 62155847 | 62632404 | 476 558 | ALB | 6 (30) | 62161330 | 16 400 | ALB |
| 18 | 14 | 48484014 | 48821243 | 337 230 | CCND1 | 26 (7) | 48641914 | 60 162 | CCND1 |
| 25 | 15 | 34458017 | 34601125 | 143 109 | EGR3 | 12 (14) | 34589168 | 7 314 | MIR320 |
| Chr . | UNC integration clusters . | Queen’s TES CIS . | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Hits . | Start . | End . | Width . | Gene∗ . | CIS (no. of events) . | Mean locus . | Width . | Gene∗ . | |
| 4 | 7 | 14530145 | 14924592 | 39 448 | EGR2 | 7 (26) | 14746173 | 72 601 | MIR1296 |
| 4 | 26 | 39589684 | 39589877 | 194 | DUSP1 | nr | nr | nr | nr |
| 13 | 5 | 62155847 | 62632404 | 476 558 | ALB | 6 (30) | 62161330 | 16 400 | ALB |
| 18 | 14 | 48484014 | 48821243 | 337 230 | CCND1 | 26 (7) | 48641914 | 60 162 | CCND1 |
| 25 | 15 | 34458017 | 34601125 | 143 109 | EGR3 | 12 (14) | 34589168 | 7 314 | MIR320 |
Comparison of CIS occurring in this study to those reported by Nguyen et al.8
Chr, chromosome; Hits, number of calls against 50 random sites; nr, not represented in TES CIS data set; UNC, University of North Carolina.
Differences in gene annotations are noted in these 2 analyses.
Integrations into the native F8 and CLIC2 (located ∼240 kb 5′ of F8) loci seen in these dogs may also be driven by homologous recombination and appear distinct from vector-into-vector events reported in a similar study.8 Integrations at ABCB1 and KCNIP2 are more difficult to explain. GTEx analysis of human liver shows low expression of ABCB1 and KCNIP2 and a multi-BLAST alignment showed 77 short homologies (50-190 bp) were observed between KCNIP2 and ABCB1. A single short (21 bp) area of homology was found between ABCB1 and the vector sequence. Further research is needed to better understand factors contributing to increased integration at these loci.
Overall, the influence of insertions located within genes that are oncogenic or tumorigenic in humans (MET, CCND1, MIR-1296, and MIR-320) on long-term safety is difficult to predict as there is no cancer reference for the canine genome and limited evidence that these genes are associated with tumorigenesis in dogs. We then examined IS locations relative to cancer genes, lifting over coordinates from the canine to the human genome and referencing to 7 human cancer gene datasets. We found that as the number of cancer genes in a data set increased, there was an increase in the number of integrations adjacent to these genes. However, when comparing our data to a simulated random data set, the proportion of IS proximal to the transcriptional start site of cancer genes fell within the normal distribution of the random control.
The final question we addressed was whether clonal expansion occurred following AAV vector delivery. A report by Nguyen et al noted FVIII elevations in 2 dogs 4 years after rAAV-FVIII treatment.8 Molecular analyses demonstrated expansion of cells containing IS, close to cancer-associated genes in the animals with rising FVIII levels and in 4 other dogs. We did not see similar elevations in FVIII activity in this study.2 Using orthogonal strategies to quantify IS abundance, we demonstrated that in 10 of 16 samples the top-10 most frequent IS represented on average 1.59% of the total IS. In the remaining 6 samples, the top-10 IS represented on average, 6.03% of the total integrations. These results indicate that whereas IS abundance is variable across different dogs and samples, no obvious clonal dominance was seen in the liver for most samples. Two biopsies exhibited integration frequencies >30% of total IS sequences, a threshold set for clonality in retroviral integration studies. However, this threshold for clonality has not been investigated in solid tissues or nonretroviral studies and its applicability to AAV gene therapy has not been validated.23 Within these studies, we cannot rule out whether clonal growth occurred due to natural liver growth or changes with age (eg, nodular hyperplasia). Whether higher clonal abundance occurred in areas of nodular hyperplasia is an active area of investigation and outside the scope of this manuscript. It should be noted that clonal dominance does not directly correlate with adverse events, as shown in the RT-PCR data and lack of tumorigenesis seen in both studies.2,8
In summary, our study demonstrates the nature of AAV vector structures a decade after administration is complex. Most intact vectors were episomal and appear to be responsible for long-term transgene expression. Variable levels of IS abundance were seen, but with no clear evidence for dominant clonal evolution. These results must be considered in the context of a small study population and limited biopsy material. These results provide further justification for ongoing investigation of AAV vector fate and function to enhance informed decisions on the long-term safety and efficacy of AAV gene therapy.
Acknowledgments
This study was supported in part by a Canadian Institutes for Health Research grant (Foundation Grant FDN 154285), and by a grant from BioMarin Pharmaceutical. The authors thank Evan Witt for overseeing the upload of genomic data to Gene Expression Omnibus.
This study was conducted in memoriam of Manfred Schmidt, a brilliant pioneer in the cell and gene therapy field and a truly exceptional colleague.
Authorship
Contribution: D.L., P.B., and S.F. designed the research study; L.H., A.P., and A.W. cared for the animals in the study; P.B., D.H., S.F., and D.L. analyzed the data; P.B., S.F., and D.L. wrote the first draft of the paper; L.L.S. revised the manuscript; M.F., C-R.S., and I.G.-F. conducted and interpreted experiments; S.A., H.T., C.S., L.Y., and C.W. performed the bioinformatic analyses; and all authors reviewed and critically edited the manuscript.
Conflict-of-interest disclosure: S.F., C-R.S., C.S., and C.W. are employees and stockholders of BioMarin Pharmaceutical Inc. P.B. has received research support from BioMarin and consulting fees or honoraria from BioMarin, Octapharma, Novo Nordisk, CSL Behring, Pfizer, and the Institute for Nursing and Medication Education. D.L. has received research support from BioMarin, CSL Behring, and Sanofi and has received consulting fees or honoraria from BioMarin, CSL Behring, Novo Nordisk, Pfizer, and Sanofi. L.L.S. has received consulting fees from BioMarin. H.T. and I.G.-F. were employees of ProtaGene CGT GmbH. S.A. and L.Y. are employees of ProtaGene CGT GmbH. M.F. is an employee of ProtaGene US Inc. The remaining authors declare no competing financial interests.
Correspondence: David Lillicrap, Department of Pathology and Molecular Medicine, Queen's University, Kingston, ON, Canada; email: david.lillicrap@queensu.ca.
References
Author notes
The target enrichment sequencing and linear amplification-mediated polymerase chain reaction data is available at the NIH Sequence Read Archive (BioProject ID: PRJNA1074750; https://www.ncbi.nlm.nih.gov/bioproject/1074750).
Data are also available upon reasonable request from the corresponding author, David Lillicrap (david.lillicrap@queensu.ca).
The online version of this article contains a data supplement.
There is a Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.





This feature is available to Subscribers Only
Sign In or Create an Account Close Modal