Iron-mediated induction of bone morphogenetic protein (BMP)6 expression by liver endothelial cells is essential for iron homeostasis regulation. We used multiple dietary and genetic mouse cohorts to demonstrate a minor functional role for the metal-ion transporter ZIP8 in regulating BMP6 expression under high-iron conditions.
TO THE EDITOR:
The iron-regulatory hormone hepcidin functions by degrading its receptor ferroportin, preventing iron release to plasma.1 Hepcidin is suppressed by pregnancy and erythropoiesis to increase iron bioavailability, and it is induced by iron and infection/inflammation to limit iron bioavailability.2-4 Iron uses the bone morphogenetic protein (BMP)–SMAD pathway to regulate hepcidin transcription,5,6 mainly via BMP2 and BMP6 ligands, which originate in liver endothelial cells (LECs)7-12 and likely function as a heterodimer.13 BMP6 is potently induced by iron and is considered the rate-limiting ligand.8 LEC culture studies demonstrate a cell-autonomous role for iron in Bmp6 transcriptional regulation.14-16 Mechanistically, oxidative stress pathways, including nuclear factor, erythroid 2 like 2 (NRF2) and Jun proto-oncogene, AP-1 transcription factor subunit (c-JUN),14,15,17 are implicated in iron-mediated BMP6 regulation. However, it remains unknown how LECs acquire iron to activate these pathways. We and others demonstrated that transferrin-bound iron and endothelial transferrin receptor 1 (TFR1; encoded by Tfrc) play a minor role in BMP6 regulation only under low-iron conditions.16,17 In contrast, non–transferrin-bound iron (NTBI), which appears when the plasma transferrin-binding capacity is exceeded, is reported to play a dominant role in BMP6 regulation.17 Notably, the metal-ion transporter Slc39a8, encoding ZRT/IRT-like protein 8 (ZIP8), is abundantly expressed in LECs compared with other liver cell populations.18,19 Moreover, single-cell RNA sequencing (RNA-Seq) from livers of dietary iron-loaded mice suggested that Slc39a8 is upregulated by iron in liver sinusoidal endothelial cells.17 This prompted the identification of ZIP8 as the lead candidate for endothelial NTBI uptake to regulate BMP6.17,20 Yet, this finding was based on data from 1 mouse per group and was not validated. Here, we examined whether ZIP8 is regulated by iron in LECs using multiple dietary iron-loaded mouse models, and we tested its functional contribution to BMP6 and iron homeostasis regulation using knockout mice.
Animal protocols were approved by the Institutional Animal Care and Use Committee at Massachusetts General Hospital. Mouse strains included wild-type 129S6 or C57BL/6. We generated endothelial-specific single Slc39a8 knockout (Slc39a8-Stab2-Cre+), double Slc39a8 and Tfrc knockout (Slc39a8-Tfrc-Stab2-Cre+), and littermate Cre– controls by mating Slc39a8 floxed (Taconic, 11296), Tfrc floxed (Jackson, 028363), and Stabilin-2-Cre+ transgenic mice.21 Mice were fed standard chow (LabDiet, 380-ppm iron) or matched purified diets (Research Diets or Envigo Teklad) containing 2- to 6-ppm background iron, 37- to 48-ppm ferric iron citrate, or 10 000- to 20 000-ppm carbonyl iron. LECs were isolated by fluorescence- or magnetic-activated cell sorting.14-16 RNA-Seq and quantitative proteomics were performed previously,15 with data sets available in public repositories.15 Quantitative reverse transcription polymerase chain reaction (qRT-PCR) and western blots were performed as described16 using primers and antibodies in supplemental Tables 1 and 2 (available on the Blood website). Serum and tissue nonheme iron were measured by colorimetric assay.16,22 Data are presented as individual values, with bars representing mean ± SEM. Normality was determined by the Shapiro-Wilk test and statistical comparisons by the 2-tailed Student t-test, the Mann-Whitney U test, or the 2-way analysis of variance using Prism 10 (GraphPad). P < .05 was considered significant.
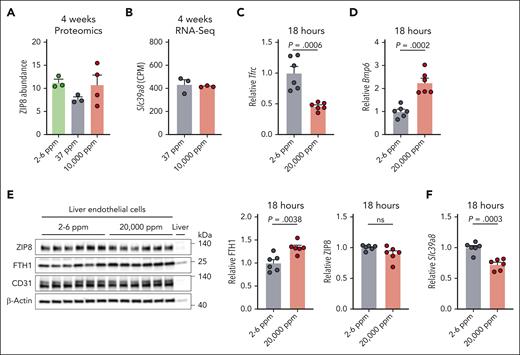
To explore whether iron regulates ZIP8 expression in LECs, as in other systems,23 we analyzed quantitative proteomics data sets of LECs isolated from mice fed iron-deficient (2- to 6-ppm), iron-sufficient (37-ppm), or high-iron (10 000-ppm) diets for 4 weeks.15 We also analyzed RNA-Seq data sets of LECs from the iron-sufficient and high-iron diet groups.15 Neither ZIP8 protein (Figure 1A) nor Slc39a8 transcript abundance (Figure 1B) significantly differed among diet groups, indicating a lack of regulation in chronically iron-deficient or iron-loaded conditions. A lack of LEC Slc39a8 mRNA induction by iron was also confirmed by qRT-PCR in an independent mouse cohort (supplemental Figure 1A-B). To determine whether iron acutely regulates Slc39a8/ZIP8, we analyzed LEC Slc39a8 transcript and ZIP8 protein expression from mice fed the iron-deficient diet for 1 week, followed by a 20 000-ppm high-iron diet for 18 hours. This feeding scheme was previously reported to induce Slc39a8 expression in liver sinusoidal endothelial cells by single-cell RNA-Seq.17 The high-iron diet loaded LECs with iron as shown by decreased Tfrc mRNA, which is inversely regulated by iron, and increased Bmp6 mRNA and ferritin protein, which are induced by iron (Figure 1C-E). However, neither Slc39a8 transcript nor ZIP8 protein expression was induced by the high-iron diet (Figure 1E-F). A lack of LEC Slc39a8 mRNA induction by acute iron loading was also confirmed in an independent cohort (supplemental Figure 1C-D). These data show that LEC Slc39a8/ZIP8 is not upregulated by iron at the transcript or protein level.
Liver endothelial expression of metal-ion transporter Slc39a8 (encoding ZIP8) is not regulated by dietary iron. (A-B) Three-week-old wild-type 129S6 male mice were fed a purified iron-deficient diet (2-6 ppm iron as background iron), iron-sufficient diet (37 ppm iron as ferric citrate), or high-iron diet (10 000 ppm carbonyl iron) for 4 weeks (n = 3-4/diet). Liver endothelial cells were isolated by fluorescence-activated cell sorting and analyzed by (A) mass spectrometry–based quantitative proteomics for abundance of ZIP8 protein or (B) RNA-seq for Slc39a8 mRNA expression in counts per million (CPM). Only cells from the iron-sufficient and high-iron diet groups were analyzed by RNA-Seq. (C-F) Seven-week-old C57BL6/J female mice were fed a purified iron-deficient diet for 1 week, then switched to a high-diet (20 000 ppm carbonyl iron) for 18 hours (n = 6/diet). Endothelial cells were isolated from livers by magnetic-activated cell sorting and analyzed for (C) Tfrc or (D) Bmp6 relative to Rpl19 mRNA expression by qRT-PCR, (E) ferritin heavy chain 1 (FTH1) and ZIP8 protein expression relative to ß-actin by western blot and chemiluminescence quantitation, and (F) Slc39a8 expression relative to Rpl19 by qRT-PCR. Bar graphs represent mean ± SEM, with individual points indicating the number of animals. Statistical differences between groups were determined by the 2-tailed Student t-test for normally distributed values or the Mann-Whitney U test for nonnormally distributed values. ns, not significant.
Liver endothelial expression of metal-ion transporter Slc39a8 (encoding ZIP8) is not regulated by dietary iron. (A-B) Three-week-old wild-type 129S6 male mice were fed a purified iron-deficient diet (2-6 ppm iron as background iron), iron-sufficient diet (37 ppm iron as ferric citrate), or high-iron diet (10 000 ppm carbonyl iron) for 4 weeks (n = 3-4/diet). Liver endothelial cells were isolated by fluorescence-activated cell sorting and analyzed by (A) mass spectrometry–based quantitative proteomics for abundance of ZIP8 protein or (B) RNA-seq for Slc39a8 mRNA expression in counts per million (CPM). Only cells from the iron-sufficient and high-iron diet groups were analyzed by RNA-Seq. (C-F) Seven-week-old C57BL6/J female mice were fed a purified iron-deficient diet for 1 week, then switched to a high-diet (20 000 ppm carbonyl iron) for 18 hours (n = 6/diet). Endothelial cells were isolated from livers by magnetic-activated cell sorting and analyzed for (C) Tfrc or (D) Bmp6 relative to Rpl19 mRNA expression by qRT-PCR, (E) ferritin heavy chain 1 (FTH1) and ZIP8 protein expression relative to ß-actin by western blot and chemiluminescence quantitation, and (F) Slc39a8 expression relative to Rpl19 by qRT-PCR. Bar graphs represent mean ± SEM, with individual points indicating the number of animals. Statistical differences between groups were determined by the 2-tailed Student t-test for normally distributed values or the Mann-Whitney U test for nonnormally distributed values. ns, not significant.
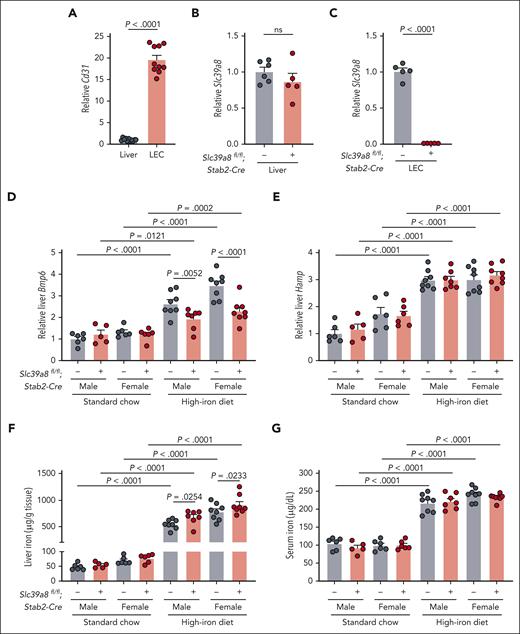
Although dietary iron failed to regulate LEC ZIP8, this does not exclude a functional role for ZIP8 in LEC iron uptake and sensing. To determine whether ZIP8 functionally contributes to Bmp6 and iron homeostasis regulation in vivo, we generated mice lacking endothelial Slc39a8 using the previously validated Stabilin-2-Cre.16,21 Efficiency and specificity were validated by comparing Slc39a8 expression in whole liver and isolated LECs, which were confirmed by enriched Cd31 expression (Figure 2A). Although Slc39a8 expression was similar in whole liver, it was >99% reduced in LECs from Slc39a8-Stab2-Cre+ mice (Figure 2B-C).
Endothelial Slc39a8-deficient mice have altered liver Bmp6 expression when fed a high-iron diet. Liver endothelial cells (LECs) were isolated from Slc39a8fl/fl;Stab2-Cre+ and Cre– mice by magnetic separation (n = 5-6/group) and analyzed for enrichment by expression of endothelial-specific marker (A) Cd31 compared with whole liver, and expression of (B-C) Slc39a8 relative to Rpl19 by qRT-PCR in whole liver and endothelial cells, respectively. In (A), cells from Cre+ and Cre– mice were combined for enrichment analysis for a total of n = 10 to 11 per group. (D-G) Five-week-old male (n = 5-8/group) and female (n = 6-8/group) Slc39a8fl/fl;Stab2-Cre+ and littermate Cre– mice were fed standard rodent chow (380 ppm iron) or purified high-iron diet (10 000 ppm iron) for 3 weeks and analyzed at 8 weeks for (D) hepatic expression of Bmp6 and (E) Hamp relative to Rpl19 by qRT-PCR, (F) liver iron, and (G) serum iron concentration. Bar graphs represent mean ± SEM, with individual points indicating the number of animals. Statistical differences were determined by (A-C) the 2-tailed Student t test for normally distributed values or (D-G) the 2-way analysis of variance followed by the 2-stage step-up method of the Benjamini, Krieger, and Yekutieli test to correct for multiple comparisons by controlling the false discovery rate (Q = 0.05). ns, not significant.
Endothelial Slc39a8-deficient mice have altered liver Bmp6 expression when fed a high-iron diet. Liver endothelial cells (LECs) were isolated from Slc39a8fl/fl;Stab2-Cre+ and Cre– mice by magnetic separation (n = 5-6/group) and analyzed for enrichment by expression of endothelial-specific marker (A) Cd31 compared with whole liver, and expression of (B-C) Slc39a8 relative to Rpl19 by qRT-PCR in whole liver and endothelial cells, respectively. In (A), cells from Cre+ and Cre– mice were combined for enrichment analysis for a total of n = 10 to 11 per group. (D-G) Five-week-old male (n = 5-8/group) and female (n = 6-8/group) Slc39a8fl/fl;Stab2-Cre+ and littermate Cre– mice were fed standard rodent chow (380 ppm iron) or purified high-iron diet (10 000 ppm iron) for 3 weeks and analyzed at 8 weeks for (D) hepatic expression of Bmp6 and (E) Hamp relative to Rpl19 by qRT-PCR, (F) liver iron, and (G) serum iron concentration. Bar graphs represent mean ± SEM, with individual points indicating the number of animals. Statistical differences were determined by (A-C) the 2-tailed Student t test for normally distributed values or (D-G) the 2-way analysis of variance followed by the 2-stage step-up method of the Benjamini, Krieger, and Yekutieli test to correct for multiple comparisons by controlling the false discovery rate (Q = 0.05). ns, not significant.
Slc39a8-Stab2-Cre+ and littermate Cre– mice were evaluated for Bmp6 expression and iron phenotype after feeding standard chow (380-ppm iron) or a high-iron diet (10 000-ppm iron) for 3 weeks. In chow-fed mice, we did not observe any differences between genotypes in total liver or LEC Bmp6 expression, hepcidin expression, liver iron, serum iron, or other iron-related parameters (Figure 2D-G; supplemental Figure 2A-F). The high-iron diet increased serum iron, increased total liver iron, and caused expected changes in surrogate measures of iron loading in LECs vs genotype-matched chow-fed mice (Figure 2F-G; supplemental Figure 2C-F). Although the high-iron diet induced total liver and LEC Bmp6 in both genotypes, total liver and LEC Bmp6 was lower in high-iron diet-fed Slc39a8-Stab2-Cre+ mice vs Cre– mice (Figure 2D; supplemental Figure 2A). This corresponded to lower levels of NRF2 targets (Gclc, Nqo1) in high-iron diet Slc39a8-Stab2-Cre+ vs Cre– LECs, although surrogate measures of iron-regulatory protein activity (Tfrc, ferritin) were not different (supplemental Figure 2C-F). These data suggest that Slc39a8 ablation may selectively impact iron-mediated oxidative stress pathways to blunt Bmp6 induction. Despite lower liver Bmp6 in high-iron diet Slc39a8-Stab2-Cre+ mice vs Cre– controls, there were no differences in hepcidin expression; however, total liver iron was modestly increased, suggesting that hepcidin was inappropriately low relative to total liver iron (Figure 2E-F). No other differences were seen between genotypes for serum iron (Figure 2G), extrahepatic tissue iron (supplemental Figure 3A-D), or LEC Bmp2 (supplemental Figure 2B). We also considered transferrin-bound iron as a signal for Bmp6 regulation. Although TFR1 is redundant under iron-replete conditions when ZIP8 is present,16,17 TFR1 function may become more apparent when ZIP8 is absent. We therefore analyzed littermate double endothelial Slc39a8-Tfrc-deficient mice and Cre– controls fed standard chow. We did not observe any differences in Bmp6 or hepcidin expression, serum iron, or liver iron between genotypes (supplemental Figure 4A-D).
Together, our data in multiple mouse models demonstrate that endothelial ZIP8 has a minor role in iron sensing and Bmp6 regulation under high-iron conditions. Analogous to TFR1, ZIP8 is mainly redundant with other iron transport mechanism(s). Previous studies suggest that the NTBI transporter ZIP14 unlikely has a major role, because global Slc39a14 knockout mice exhibit increased liver Bmp6 together with nonparenchymal iron loading.24 Additional possibilities are an unidentified NTBI transporter or a transporter-independent uptake mechanism. Because ferritin also delivers iron to LECs to induce Bmp6,16 ferritin-bound iron may also be a major iron source for BMP6 regulation. Finally, although not previously explored, iron exporters may also contribute. Identifying the mechanisms responsible for altering LEC iron content to control BMP6 expression is essential for understanding how iron levels are sensed to regulate body iron homeostasis.
Acknowledgments
The authors thank the Harvard Stem Cell Institute–Center for Regenerative Medicine Flow Cytometry Core Facility for performing fluorescence-activated cell sorting and the MGH NextGen Sequencing Core for performing RNA sequencing. The authors thank Airie Kim (UCLA) for providing the ZIP8 antibody.
This study was supported by the National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases grants T32-DK007540 (to A.L.F.), R01-DK087727 and R01-DK128068 (to J.L.B.), and R01-DK124384 (to J.D.M.); and the Patricia and Scott Eston Massachusetts General Hospital Research Scholar Award (to J.L.B.).
Authorship
Contribution: A.L.F. and S.P. designed and performed experiments, analyzed data, and wrote the manuscript; C.-Y.W., X.X., G.A.M., and A.S. performed experiments and analyzed data; J.A.P. and J.D.M. performed proteomics and analyzed data; J.L.B. conceived and designed the project and wrote the manuscript; and all authors commented on the manuscript.
Conflict-of-interest disclosure: J.L.B. had, during this research, equity interest in Ferrumax Pharmaceuticals, a company focused on targeting repulsive guidance molecule proteins (including hemojuvelin) and bone morphogenetic protein (transforming growth factor-β) superfamily signaling as hepcidin-modulating agents for the treatment of anemia and other iron disorders. J.L.B.’s interests were reviewed and are managed by Massachusetts General Hospital and Mass General Brigham in accordance with their conflict-of-interest policies. C.-Y.W. is currently employed at Keros Therapeutics (Lexington, MA). The remaining authors declare no competing financial interests.
The current affiliation for C.-Y.W. is Keros Therapeutics, Lexington, MA.
Correspondence: Jodie L. Babitt, Nephrology Division and Endocrine Unit, Massachusetts General Hospital, Harvard Medical School, Thier Research Building, 1123A, 50 Blossom St, Boston, MA 02114; email: babitt.jodie@mgh.harvard.edu.
References
Author notes
A.L.F. and S.P. contributed equally to this study.
RNA-sequencing data have been deposited in the National Center for Biotechnology Information Gene Expression Omnibus (GEO) and are accessible through the GEO Series accession number GSE223871. Mass spectrometry proteomics data have been deposited to the ProteomeXchange Consortium via the Proteomics Identification Database (PRIDE) partner repository with the data set identifier PXD039830.
Data are available on request from the corresponding author: Jodie L. Babitt (babitt.jodie@mgh.harvard.edu).
The online version of this article contains a data supplement.
There is a Blood Commentary on this article in this issue.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal