In this issue of Blood, Chalandon et al1 report that attenuation of postremission chemotherapy in patients with Philadelphia chromosome-positive (Ph+) acute lymphoblastic leukemia (ALL) treated with nilotinib leads to increased relapse. Ph+ ALL is a formerly dreaded subtype of ALL in adults as resistance to conventional chemotherapy resulted in miserable survival rates, especially in the absence of allogeneic hematopoietic stem cell transplant (alloHSCT).2 In 2001, “the activity of a specific inhibitor of the BCR-ABL tyrosine kinase” was reported3 and revolutionized the treatment and outcomes of Ph+ ALL. The specific tyrosine kinase inhibitor (TKI) (imatinib) was swiftly incorporated into conventional chemotherapy ALL regimens increasing responses, rates of transplantation, and overall survival.4
The trial reported here, Group for Research on Adult Acute Lymphoblastic Leukemia Philadelphia positive (GRAAPH)-2014, builds from the preceding GRAAPH-2005 trial, which proved that powerful chemotherapy is not needed to achieve remission in patients treated with imatinib.5 In GRAAPH-2005, patients randomized to imatinib plus reduced-intensity induction chemotherapy had lower induction mortality and higher complete remission rates compared with patients randomized to imatinib plus “standard” (intensive) induction chemotherapy.5 Subsequently, patients received consolidation chemotherapy followed by alloHSCT with a 4-year survival of 48%, better when compared with survival before the imatinib era (∼20%).4
To further improve survival, clinical investigation has focused on improving the depth and durability of response and decreasing therapy-related mortality. Second- and third-generation TKIs have now supplanted imatinib, with deeper remissions and improved survival reported in patients also receiving chemotherapy and often alloHSCT.6-8 The GRAAPH-2014 investigators focused on determining whether continuous exposure to the second-generation TKI, nilotinib, would allow further lowering of chemotherapy intensity in the consolidation phase of treatment without impairing efficacy.
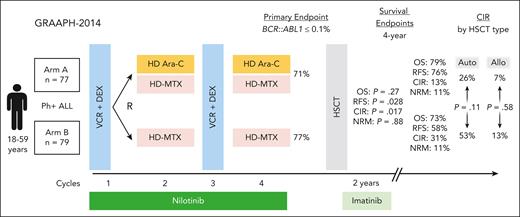
GRAAPH-2014 enrolled patients aged 18 to 59 years with newly diagnosed Ph+ ALL across 79 centers in France, Belgium, and Switzerland. All patients received an initial low-intensity induction and then were randomly assigned to receive or not receive 2 cycles of high-dose cytarabine consolidation along with continuous nilotinib (see figure). The study was designed as a noninferiority trial with the primary end point achievement of major molecular response (BCR::ABL1 ≤ 0.1%) after 4 cycles of therapy (end consolidation). Subsequently patients received alloHSCT, with autoHSCT permitted if BCR::ABL1 ≤ 0.1%.
GRAAPH-2014 trial schema. DEX, dexamethasone; HD Ara-C, high-dose cytarabine; HD MTX, high-dose methotrexate; NRM, nonrelapse mortality; OS, overall survival; RFS, relapse-free survival; VCR, vincristine.
GRAAPH-2014 trial schema. DEX, dexamethasone; HD Ara-C, high-dose cytarabine; HD MTX, high-dose methotrexate; NRM, nonrelapse mortality; OS, overall survival; RFS, relapse-free survival; VCR, vincristine.
The primary end point was achieved at the same rate in both arms (71% [arm A, cytarabine] vs 77% [arm B, no cytarabine], P = .0016, noninferiority established). However, despite achieving its primary end point, the study was stopped early (after accrual of 156 of 265 planned patients) due to an excess of relapse in patients who did not receive cytarabine (arm B) compared with those who did (arm A) with 4-year cumulative incidence of relapse (CIR) of 31% vs 13% (P = .017). T315 was the most detected resistance mutation. The authors reported that the increase in relapse was not evenly distributed, with higher CIR rates without cytarabine (arm B) seen in patients consolidated with autoHSCT (52% [arm B] vs 26% [arm A], P = .11), but comparable in those consolidated with alloHSCT (13% [arm B] vs 7% [arm A], P = .58), although statistical significance was not achieved. The conclusion is that in the context of a second-generation TKI, cytarabine consolidation is associated with a lower risk of relapse, particularly in the absence of alloHSCT. Of note, alloHSCT was associated with a longer relapse-free survival in a time-dependent analysis (hazard ratio 0.33, P = .0001), which was confirmed in arm B (no cytarabine), but not in arm A (cytarabine).
So, what do we take away from this study? First, outcomes for adults with Ph+ increased by a whopping 30 percentage points over a decade: from 48% (GRAAPH-2005) to 79.4% (GRAAPH-2014) 4-year overall survival. This result confirms the effectiveness of treatment approaches based on a second-generation TKI in combination with intensive chemotherapy and/or alloHSCT. Second, although most patients in this study received intensive consolidation chemotherapy and/or alloHSCT, tolerability was good with early mortality <5% and nonrelapse mortality 11% at 4 years. Third, relapses in the central nervous system (CNS) were conspicuously rare (only 1 in each arm), highlighting the effectiveness of the GRAAPH approach in preventing CNS disease and the benefit of CNS-penetrating chemotherapy. Fourth, as in other studies, the T315 mutation characterized many relapses, supporting the study of ponatinib in Ph+ ALL. Finally, although alloHSCT was shown to be beneficial, that benefit was not apparent in patients who received cytarabine consolidation, leaving open the debate about the necessity of alloHSCT in patients responding optimally to TKI plus intensive consolidation chemotherapy.
Despite the progress clearly demonstrated by Chalandon et al,5 it is unlikely (as the authors themselves acknowledge) that the GRAAPH-2014 regimen will become a standard. Blinatumomab, a CD19-CD3 bispecific T-cell engager, has burst on the scene as a remarkably potent, well-tolerated consolidation approach in Ph+ ALL. The recently published long-term results of the Gruppo Italiano Malattie Ematologiche dell'Adulto (GIMEMA) LAL2116 D-ALBA study show durable remissions among patients induced with dasatinib and consolidated with dasatinib plus blinatumomab, including many who did not receive any chemotherapy and were not allografted.9 Ponatinib reduces T315I relapses and in combination with blinatumomab may be particularly powerful, hopefully even among those with high-risk IKZF1 plus abnormalities.6,8,10 A notable advantage of a blinatumomab-based approach is tolerability in patients >60 years of age who were not eligible for this study, an important feature given the older age distribution of Ph+ ALL. A specific concern regarding blinatumomab-based approaches is the frequency of CNS relapses, and the best approach to CNS prophylaxis in the context of blinatumomab needs consideration.
There are now many effective ingredients to treat Ph+ ALL, a situation unfathomable not so long ago. The challenge now is to design just the right recipe(s). The results of ongoing and planned trials will help refine the treatment formula for this now favorable subtype of ALL.
Conflict-of-interest disclosure: M.R.L. reports research funding from Novartis and AbbVie and honoraria from Pfizer, Novartis, Jazz, and Kite.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal