In this issue of Blood, Keenan et al tease apart the impact of combined JAK1 and JAK2 inhibition vs selective inhibition on downstream signaling and disease modification in primary and secondary hemophagocytic lymphohistiocytosis (HLH) mouse models.1 Their work provides valuable insights that may become relevant for clinicians grappling with intricate decision-making in patients with HLH.
HLH is a hyperinflammatory syndrome characterized by T-cell activation, enhanced JAK/STAT signaling and interferon gamma (IFN-γ) response, and uncontrolled mononuclear phagocyte activation.2 Clinically, it presents in 2 forms: primary, driven by mutations affecting cytotoxic T-cell function; and secondary, due to various underlying conditions, such as malignancies and rheumatological diseases.3
In leveraging the activated JAK/STAT signaling demonstrated in HLH in preclinical studies4,5 and clinical reports,6 broad JAK inhibition using ruxolitinib has been proposed as a way to control the characteristic hyperinflammation seen in this condition. However, this approach may not be ideal given the potential myelosuppressive effects of JAK inhibitors among patients in whom cytopenias are almost always present. Fine tuning to achieve selective JAK inhibition may be a way to circumvent this problem.
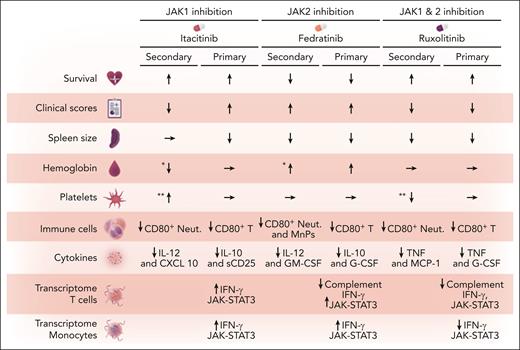
After adjustments in dose using STAT1 phosphorylation assays, Keenan et al explored the efficacy of combined JAK1/2 inhibition using ruxolitinib compared with selective JAK1 inhibition with itacitinib and selective JAK2 inhibition with fedratinib. Their findings reveal a variable and mixed response pattern (see figure).
Effects of JAK inhibition in HLH mouse models. The figure illustrates the impact of JAK inhibition in both primary and secondary HLH mouse models compared with controls. The primary model uses perforin-deficient−/− mice challenged by lymphocytic choriomeningitis virus, whereas the secondary model is induced by CpG+ IL-10 receptor-blocking antibody. Key observations include changes in various immune cell populations and cytokine levels. G-CSF, granulocyte colony-stimulating factor; GM-CSF, granulocyte-macrophage colony-stimulating factor; IL, interleukin; MnPs, mononuclear phagocytes (Ly6C+ of CD11b+ cells); Neut, CD80+ neutrophils; sCD25, soluble CD25. ∗Difference between itacitinib and fedratinib treatments. ∗∗Difference between itacitinib and ruxolitinib treatments.
Effects of JAK inhibition in HLH mouse models. The figure illustrates the impact of JAK inhibition in both primary and secondary HLH mouse models compared with controls. The primary model uses perforin-deficient−/− mice challenged by lymphocytic choriomeningitis virus, whereas the secondary model is induced by CpG+ IL-10 receptor-blocking antibody. Key observations include changes in various immune cell populations and cytokine levels. G-CSF, granulocyte colony-stimulating factor; GM-CSF, granulocyte-macrophage colony-stimulating factor; IL, interleukin; MnPs, mononuclear phagocytes (Ly6C+ of CD11b+ cells); Neut, CD80+ neutrophils; sCD25, soluble CD25. ∗Difference between itacitinib and fedratinib treatments. ∗∗Difference between itacitinib and ruxolitinib treatments.
Although JAK1 inhibition using itacitinib enhanced survival rates, improved clinical scores, and elevated platelet counts compared with ruxolitinib in the CpG+ IL-10 receptor-blocking antibody-induced secondary HLH mouse model, it failed to mitigate either splenomegaly or anemia and did not increase platelet counts compared with controls. In the primary HLH perforin-deficient−/− mouse model with lymphocytic choriomeningitis virus challenge, itacitinib exhibited partial improvements in survival and did decrease the spleen size, but it did not improve disease manifestations such as hemoglobin level and platelet counts.
When JAK2 was selectively inhibited with fedratinib, no improvement was observed in clinical scores or survival rates in either model system. However, spleen size reduction was achieved in both models when compared with control mice. In addition, in the primary HLH model, hemoglobin levels increased compared with control and itacitinib-treated mice. In the secondary HLH model, hemoglobin increased compared with itacitinib-treated mice but not control mice. Platelet counts were unchanged compared with controls in both models. Fedratinib also decreased total monocyte and neutrophil counts compared with controls in the secondary HLH model. In the primary HLH model, it significantly reduced CD8+ T-cell counts and IFN-γ production and it decreased various cytokines in both models (see figure), with a profound decrease in interleukin-10 and soluble CD25 (sCD25).
Ruxolitinib, a JAK1/2 inhibitor, demonstrated significant improvements in survival rates and clinical scores as well as spleen size reduction in both experimental models. Ruxolitinib suppressed levels of tumor necrosis factor (TNF) and monocyte chemoattractant protein-1 in the primary HLH model, and reduced TNF, sCD25, and interleukin-10 levels in the secondary HLH model. In both models, ruxolitinib-induced decreases in CD8+ T-cell counts and IFN-γ secretion were less pronounced than those achieved with fedratinib.
The investigators conducted a transcriptomic analysis of splenic CD8 T cells and monocytes collected on day 9 from the primary HLH mice to probe the significant variability in model and drug responses. Bulk RNA sequencing of sorted cells revealed distinct treatment-related transcriptional profiles in both CD8 T cells and monocytes. Ruxolitinib demonstrated the most pronounced effect on gene expression, consistently downregulating pro-inflammatory pathways to a greater extent than itacitinib or fedratinib in both cell types.
The occurrence of HLH in patients with hematological malignancies has been highlighted in recent research.7 The combined elevation in sCD25 and ferritin levels has emerged as a valuable tool for identifying these patients.8 The findings of Keenan et al may find ready translation into clinical practice. For example, treatment with ruxolitinib rather than fedratinib may be favored in patients with myelofibrosis and HLH-associated hyperinflammation. Likewise, the study suggests potential consideration of itacitinib as a treatment option for patients with secondary HLH and significant thrombocytopenia.
Keenan et al's research also raises questions: What downstream signaling events are relevant in accounting for the distinct responses to various JAK inhibitors? What factors contribute to the variability in results between the 2 HLH mouse models? Moreover, the definition of outcomes to gauge treatment efficacy must be standardized and prioritized: Should these be signaling pathway activity, cytokine levels, spleen size, hematological parameters, or survival rates? These questions highlight the complexities inherent in this field of research and underscore the need for further investigation to optimize treatment strategies.
Numerous JAK inhibitors are currently undergoing clinical development, particularly for the treatment of myeloproliferative neoplasms and autoimmune disorders. These inhibitors vary in their JAK specificities and binding affinities. Some affect noncanonical signaling pathways alongside JAK inhibition, with salutary effects on cytopenias demonstrated in myelofibrosis. For instance, momelotinib targets the activin A receptor type 1 receptor, leading to increased hemoglobin levels via a hepcidin pathway–dependent mechanism.9 Similarly, pacritinib suppresses interleukin-1 receptor–associated kinase 1 and improves myelofibrosis-related thrombocytopenia.10 The potential effects of these novel JAK inhibitors on HLH in the authors' mouse models would be intriguing to explore and could be valuable clinically.
Although fundamental biological questions are still being addressed, patients with HLH may be hopeful that international registry initiatives and ongoing clinical trials investigating novel agents, including ruxolitinib, will propel the development of more effective HLH treatments and address the challenges posed by the severe cytopenias typical of this disorder. The optimal treatment for HLH is not readily apparent, but the novel research by Keenan and colleagues offers considerable promise. Although we do not yet appear to have an ace up our sleeve, we certainly have plenty of JAKs to deal.
Conflict-of-interest disclosure: A.Z.-L. has served as a consultant for Sobi, Inc.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal