Issue Archive
Table of Contents
BLOOD COMMENTARIES
SPECIAL REPORT
Remission, treatment failure, and relapse in pediatric ALL: an international consensus of the Ponte-di-Legno Consortium
Clinical Trials & Observations
In a landmark Special Report led by Buchmann, authors from among the largest international pediatric cooperative groups report on key steps towards harmonizing definitions for complete remission, treatment failure, and relapse in patients with acute lymphoblastic leukemia (ALL). By applying these modern definitions, comparisons can be made between studies in different parts of the world and international collaborations enhanced to expedite improvements in care.
CLINICAL TRIALS AND OBSERVATIONS
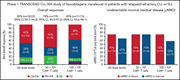
Phase 1 TRANSCEND CLL 004 study of lisocabtagene maraleucel in patients with relapsed/refractory CLL or SLL
Clinical Trials & Observations
Patients with chronic lymphocytic leukemia (CLL) or small lymphocytic lymphoma (SLL) refractory to ibrutinib and bearing unfavourable genetics have a very poor prognosis. Siddiqi and colleagues summarize the first results of a phase 1 trial of the anti-CD19 chimeric antigen receptor T-cell (CAR T) product, lisocabtagene, in 25 such patients, reporting frequent mild cytokine release syndrome, with high-grade neurological toxicity in one-fifth of the patients. Preliminary efficacy appears promising, with 10 patients achieving complete remission and nine without detectable minimal residual disease. Mature phase 2 data are needed to assess the lasting clinical benefits from this specific CAR T approach.
Disease progression and clinical outcomes in telomere biology disorders
Clinical Trials & Observations
Telomeres are specialized structures at the ends of chromosomes that maintain genomic stability. Niewisch et al document the clinical features, including bone marrow failure and survival outcomes associated with different germline genotypes of telomere biology disorders in 200 patients. They show that both genotype and inheritance pattern affect outcomes, thereby better informing risk stratification and clinical management of patients with these rare diseases.
IMMUNOBIOLOGY AND IMMUNOTHERAPY
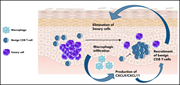
Macrophage-derived CXCL9 and CXCL11, T-cell skin homing, and disease control in mogamulizumab-treated CTCL patients
Clinical Trials & Observations
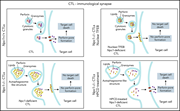
Severely impaired CTL killing is a feature of the neurological disorder Niemann-Pick disease type C1
LYMPHOID NEOPLASIA
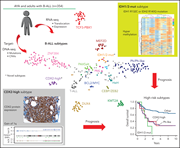
Two novel high-risk adult B-cell acute lymphoblastic leukemia subtypes with high expression of CDX2 and IDH1/2 mutations
Clinical Trials & Observations
Using comprehensive integrated genomic analyses in a cohort of 354 trial patients ages 15 to 64 years, Yasuda and colleagues define the landscape of adult Philadelphia chromosome–negative B-cell acute lymphoblastic leukemia (ALL). Two new high-risk subtypes not seen in pediatric populations are identified, one characterized by high CDX2 expression and the other by IDH1/2 mutations. These discoveries generate new questions about ALL biology and provide avenues for improved therapy for patients with very poor prognosis.
Molecular features encoded in the ctDNA reveal heterogeneity and predict outcome in high-risk aggressive B-cell lymphoma
There is excitement about the potential for circulating tumor DNA (ctDNA) assays to better inform care of patients with diffuse large B-cell lymphoma beyond existing data from tumor biopsies, imaging, and clinical estimates. In a rigorous prospective validation, Meriranta et al report on a trial-based analysis of the prognostic utility of ctDNA in the pretreatment, on-treatment, and end-of-treatment settings beyond what can be achieved with tumor biopsies and imaging. Furthermore, they highlight the concept that ctDNA is not a single assay but rather a substrate that can be informatively assayed in different dimensions according to the clinical setting.
PLATELETS AND THROMBOPOIESIS
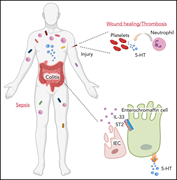
Intestinal IL-33 promotes platelet activity for neutrophil recruitment during acute inflammation
Interleukin-33 (IL-33) is expressed by gut epithelial cells and increases after tissue damage. Using murine models, Chen et al reveal that intestinal IL-33 enhances serotonin release, thereby activating platelets, perturbing hemostasis, and promoting platelet-dependent neutrophil recruitment during acute inflammation. This work establishes a new axis for endocrine-blood cell crosstalk linking local inflammation with systemic effects on hemostasis.
THROMBOSIS AND HEMOSTASIS
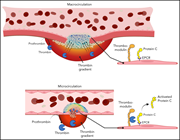
Thrombin spatial distribution determines protein C activation during hemostasis and thrombosis
Deploying both in vivo and in silico modeling, Marar and colleagues describe that the extent of protein C (PC) activation at sites of vascular injury is determined largely by the spatial distribution of thrombin activity, in conjunction with local physical conditions. These findings provide a mechanistic basis for context-dependent contributions of activated PC anticoagulant activity and its role in the regulation of thrombin generation.
Most anti-PF4 antibodies in vaccine-induced immune thrombotic thrombocytopenia are transient
Clinical Trials & Observations
Brief Report
Schönborn et al report on the natural history of platelet factor 4 (PF4)-dependent platelet-activating antibodies in 65 patients who developed COVID-19 vaccine-induced thrombotic thrombocytopenia (VITT), finding that the great majority are transitory. They also report that patients with VITT receiving a subsequent mRNA-produced vaccine do not develop new thromboses or an increase in anti-PF4 antibodies, reinforcing the safety of this approach.
TRANSPLANTATION
Blinatumomab maintenance after allogeneic hematopoietic cell transplantation for B-lineage acute lymphoblastic leukemia
Clinical Trials & Observations
LETTER TO BLOOD
The predictive value of a positive phase 2 ASH abstract for peer-reviewed publication and progression to phase 3
Clinical Trials & Observations
BLOOD WORK
ERRATUM
-
Cover Image
Cover Image
![issue cover]()
Three-dimensional reconstruction of enlarged and clustered granzyme B containing cytotoxic granules (green) in an Npc1−/− CD8 T cell. What causes these changes and how they affect the function of T cells in Npc1−/− mice and NPC1 patients are discussed in the article by Castiblanco et al on page 1833.
- PDF Icon Front MatterFront Matter
- PDF Icon Table of ContentsTable of Contents
- PDF Icon Back MatterBack Matter
- PDF Icon Editorial BoardEditorial Board
Advertisement intended for health care professionals
Email alerts
Advertisement intended for health care professionals






Do CARs finally hit the CLL road?
Clinical Trials & Observations