The study from Meriranta et al1 in this issue of Blood details circulating tumor DNA analysis from 101 patients with large B-cell lymphomas undergoing first-line therapy on the Nordic LBC-05 trial. Using a custom capture-based sequencing approach, the authors assessed plasma cell-free DNA at time points before, during, and after immunochemotherapy. The investigators extensively analyze diverse features of tumor-derived cell-free DNA molecules, including quantitative measurements of tumor DNA, dynamic measurements during and after therapy, and mutational genotypes.
In addition to uses for tumor-burden assessment and mutational profiling, the authors explore fragmentation patterns of mutant and wild-type cell-free DNA molecules across these same samples. This study meticulously assesses the clinical and prognostic significance of each of these features of cell-free DNA. Specifically, the authors convincingly demonstrate the adverse prognostic value of high circulating tumor DNA levels before therapy and detectable residual circulating tumor DNA at the end of therapy. Moreover, they demonstrate the performance of circulating tumor DNA analysis to identify tumor-specific genotypes, including associations between TP53 mutation and adverse outcomes, as well as emergence of occult MYC genomic events in relapsing disease.
The utility of circulating tumor DNA in the clinic for mutational genotyping, pretreatment prognostication, and molecular response assessment has been an important topic in the last 5 years. Initial studies used immunoglobulin high-throughput sequencing to quantify disease burden,2,3 whereas more recent studies, such as the current work by Meriranta et al, used capture-based sequencing to both quantify disease burden and identify tumor-specific mutations.4-6 This study adds to the growing body of evidence that circulating tumor DNA can provide useful prognostic information before treatment,4,7 assess molecular response at interim time points and residual disease at the end of therapy,3-6 and identify tumor-specific mutations.5,6 Furthermore, recent studies have suggested differences in the length of DNA fragments derived from tumor vs nontumor origin, with shorter DNA fragments enriched for tumor-derived molecules.8 The present study extends this finding to large B-cell lymphomas, demonstrating robust and reproducible patterns in molecular length of plasma DNA from patients with lymphoma.
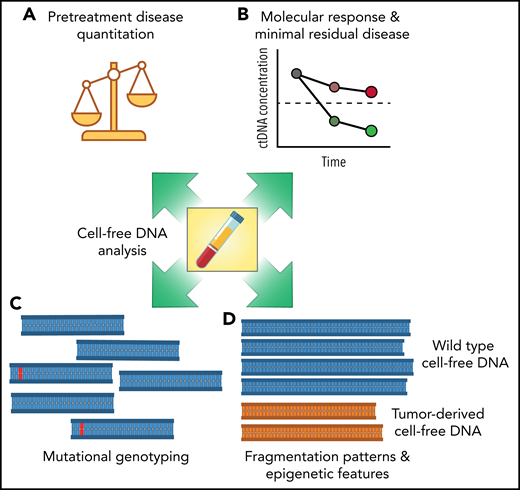
The work from Meriranta et al further clarifies the concept that circulating tumor DNA is not a single assay but rather an analyte that can reveal multiple dimensions of a tumor (see figure). Each of these features potentially exposes independent aspects of a malignancy, such as tumor burden, responsiveness to therapy, and underlying biological and molecular differences, which may serve as actionable biomarkers for targeted treatments. As the field becomes more refined, specifying which of these features are of most interest—and which assays are best suited to each application—will become important. Methods to detect and quantify pretreatment and residual tumor burden have been extensively studied previously. Technologies for quantifying circulating tumor DNA burden are therefore closest to translation, with clinical trials focused on risk-adapted therapy for diffuse large B-cell lymphoma (DLBCL) a possible path forward. Methods to identify specific mutations or mutational subtypes also show significant promise, although clinical translation will depend on the development of subtype-specific targeted therapies for treatment selection. Finally, methods to assess epigenetic tumor features in the absence of mutations, either through DNA fragmentation patterns or methylation,9,10 have been the least studied in lymphomas, although potentially have the most promise for understanding the transcriptional programming of a given patient’s disease.
Multiple dimensions of cell-free DNA in lymphomas. The study from Meriranta et al examines multiple aspects of cell-free DNA in large B-cell lymphomas, including pretreatment disease quantification (A), molecular response assessment (B), mutational genotyping (C), and DNA fragmentation patterns (D). Each feature of cell-free DNA molecules has potential for translation into the clinic as a prognostic biomarker.
Multiple dimensions of cell-free DNA in lymphomas. The study from Meriranta et al examines multiple aspects of cell-free DNA in large B-cell lymphomas, including pretreatment disease quantification (A), molecular response assessment (B), mutational genotyping (C), and DNA fragmentation patterns (D). Each feature of cell-free DNA molecules has potential for translation into the clinic as a prognostic biomarker.
It remains to be seen if the value of circulating tumor DNA in the clinic will primarily be as a “minimal residual disease” test, or whether techniques focused on characterizing the tumor genome or epigenome will become a routine part of clinical evaluation. However, each additional study further confirming and adding to the utility of liquid biopsies suggests that circulating tumor DNA analysis may become a common thread throughout a patient’s experience with large B-cell lymphoma, augmenting or even replacing standard pathology or radiographic imaging. Ultimately, prospective clinical studies will be required to define how and when this analyte should be used to improve patient outcomes. Still, with the growing body of evidence for its utility, it is likely not a matter of if, but when, circulating tumor DNA becomes part of routine evaluation of DLBCL.
Conflict-of-interest disclosure: D.M.K. has served as a consultant for Roche and Genentech, has ownership equity in Foresight Diagnostics, and has patents pending related to methods for analysis of cell free nucleic acids and methods for treatment selection based on statistical frameworks of clinical outcome.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal