Key Points
Platelet-activating anti-PF4 antibodies in VITT are transient in >90% of patients.
Likely VITT patients can safely receive a second vaccination shot with an mRNA vaccine, independent of their VITT-antibody status.
Abstract
Vaccine-induced thrombotic thrombocytopenia (VITT) is triggered by vaccination against COVID-19 with adenovirus vector vaccines (ChAdOx1 nCoV-19; Ad26.COV2-S). In this observational study, we followed VITT patients for changes in their reactivity of platelet-activating antiplatelet factor 4 (PF4) immunoglobulin G (IgG) antibodies by an anti-PF4/heparin IgG enzyme immunoassay (EIA) and a functional test for PF4-dependent, platelet-activating antibodies, and new thrombotic complications. Sixty-five VITT patients (41 females; median, 51 years; range, 18-80 years) were followed for a median of 25 weeks (range, 3-36 weeks). In 48/65 patients (73.8%; CI, 62.0% to 83.0%) the functional assay became negative. The median time to negative functional test result was 15.5 weeks (range, 5-28 weeks). In parallel, EIA optical density (OD) values decreased from median 3.12 to 1.52 (P < .0001), but seroreversion to a negative result was seen in only 14 (21.5%) patients. Five (7.5%) patients showed persistent platelet-activating antibodies and high EIA ODs for >11 weeks. None of the 29 VITT patients who received a second vaccination dose with an mRNA COVID-19 vaccine developed new thromboses or relevant increase in anti-PF4/heparin IgG EIA OD, regardless of whether PF4-dependent platelet-activating antibodies were still present. PF4-dependent platelet-activating antibodies are transient in most patients with VITT. VITT patients can safely receive a second COVID-19 mRNA-vaccine shot.
Introduction
Since March 2021, hundreds of patients have developed vaccine-induced immune thrombotic thrombocytopenia (VITT) (synonym: thrombosis and thrombocytopenia syndrome) after vaccination with the ChAdOx1 nCoV-191-3 or Ad26.COV2.S (Janssen)4,5 COVID-19 vaccine. We6 and others7-11 have identified platelet-activating anti–platelet factor 4 (PF4) immunoglobulin G (IgG) antibodies associated with VITT.
Observational studies of VITT patients indicate that clinical and laboratory features6-8 are very similar to those of heparin-induced thrombocytopenia (HIT).12-17 Typically, platelet-activating antibodies in HIT are no longer detectable in most individuals after 3 months, whereas antibodies reacting in the anti-PF4/heparin enzyme immunoassay (EIA) are often detectable for a few more weeks.13
Repeated vaccination shots are required to achieve sufficient long-term protection against COVID-19.18-20 It is unclear whether further vaccination is possible without inducing a relapse of VITT. Analogous to HIT,21 the application of a second vaccine shot might be dangerous for VITT patients if platelet-activating anti-PF4 antibodies are still present.
Here we report data on anti-PF4 antibody transience in a large cohort of patients who developed VITT after vaccination with ChAdOx1 nCoV-19 or Ad26.COV2.S, corroborating and expanding our previous observations.22
Study design
Data collection
As described,22 we enrolled all patients referred to our laboratory with clinical suspicion of VITT confirmed by a positive anti-PF4/heparin IgG (EIA) and a positive PF4-dependent platelet activation test, who gave informed consent. Serum samples were periodically referred by the treating physician. Patient characteristics were obtained during initial diagnosis; missing data were obtained by contacting patients and treating physicians via telephone or e-mail.
Assays for PF4-dependent antibodies
Results and discussion
Patients’ characteristics
Over a median follow-up of 25 weeks (range, 3-36 weeks), we studied 65 patients with serologically confirmed VITT of whom at least 2 blood samples were available: 41 females (63%); median age, 51 years (range, 18-80 years); 59 patients were initially vaccinated with ChAdOx1 nCoV-19 COVID-19 vaccine, 6 patients with the Ad26.COV.S COVID-19 Vaccine Janssen. During acute VITT, 30 patients (46.2%) developed cerebral venous sinus thrombosis, 10 (15.4%) developed splanchnic vein thrombosis, 19 (29.2%) developed pulmonary embolism, 13 (20.0%) developed deep vein thrombosis, and 4 patients developed arterial thrombosis (6.2%). Twenty-two (33.8%) patients showed multiple locations of thrombosis; 8 (12.3%) patients had typical pre-VITT24 with headache and thrombocytopenia but no thrombosis. The median time between the day of vaccination and onset of VITT-associated symptoms was 9 days (range, 4-30 days). Until 25 November 2021, 53 patients had been followed for >20 weeks.
Transience of platelet-activating antibodies
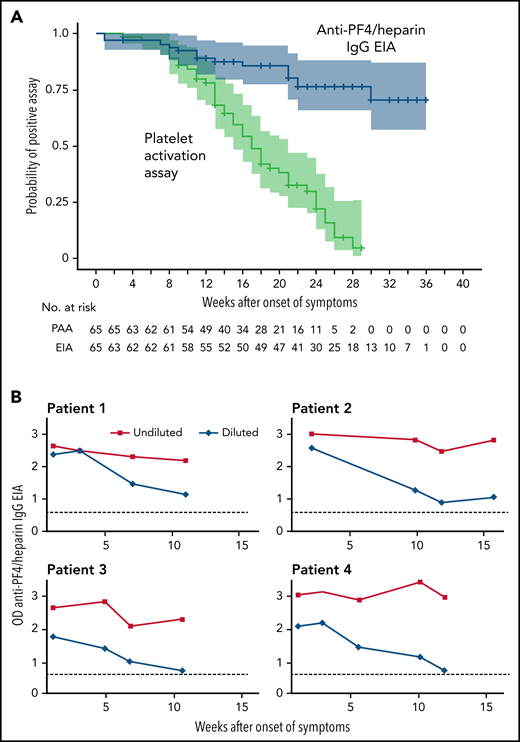
The platelet-activation assay became negative in 48/65 patients (73.8%; CI, 62.0% to 83.0%) with a median time to a negative test result of 15.5 weeks (range, 5-28 weeks) (Figure 1A). This is somewhat longer than the 12 weeks anticipated based on the first series of patients.22 In addition, the persistence of antibodies shows wide individual variability. Of the 53 patients with a follow-up of >20 weeks after onset of VITT, 33 patients (62.3%; 95% CI, 48.8% to 74.1%) showed a negative platelet-activation assay within 20 weeks, 11 patients (20.8%; 95% CI, 12.0% to 33.5%) showed a negative platelet-activation assay within 20-30 weeks. Nine of 53 patients (17.0%; 95% CI, 9.2% to 29.2%) showed platelet-activating antibodies until the last available sample. We, therefore, recommend to follow VITT patients for at least 20 weeks after acute VITT to recognize those with a persisting prothrombotic state. When anticoagulation is stopped after several months, monitoring of D-dimer levels may indicate patients at risk for recurrent thrombosis. This is especially relevant in patients whose platelet count is still reduced. Follow-up serological studies (EIA, platelet-activation assay) may then confirm or rule out persisting platelet-activating anti-PF4 antibodies.
Dynamic of anti-PF4 antibody response. (A) Kaplan-Meier Analysis of the proportion of patients with an anti-PF4/heparin IgG EIA optical density (OD) >0.5 and patients with a positive platelet activation assay after VITT (n = 65). The time (in weeks) to a negative anti-PF4/heparin IgG EIA (OD <0.5) and to a negative test by the platelet activation assay is shown. All patients initially had a positive assay for PF4-dependent platelet-activating antibodies and were repeatedly tested (median follow-up of 25 weeks; range, 3-36 weeks). Patients at risk are shown under the graph. Antibody levels in the anti-PF4/heparin IgG EIA decreased over the time of follow-up, but only in 14 patients the OD fell below the cut off OD <0.5. (B) ODs of the anti-PF4/heparin IgG EIA (antigen assay) of 4 VITT patients with persistently high ODs over time of undiluted and diluted serial samples. The OD of the anti-PF4/heparin IgG EIA remained high with values of >2.0 over 11 weeks. After dilution of the samples (1:4000 in patients 1-3; 1:2000 in patient 4), ODs again declined, showing that the persistently high OD values were caused by the low dynamic range of the EIA at high antibody concentrations. The dotted line (...) shows the cutoff OD <0.5.
Dynamic of anti-PF4 antibody response. (A) Kaplan-Meier Analysis of the proportion of patients with an anti-PF4/heparin IgG EIA optical density (OD) >0.5 and patients with a positive platelet activation assay after VITT (n = 65). The time (in weeks) to a negative anti-PF4/heparin IgG EIA (OD <0.5) and to a negative test by the platelet activation assay is shown. All patients initially had a positive assay for PF4-dependent platelet-activating antibodies and were repeatedly tested (median follow-up of 25 weeks; range, 3-36 weeks). Patients at risk are shown under the graph. Antibody levels in the anti-PF4/heparin IgG EIA decreased over the time of follow-up, but only in 14 patients the OD fell below the cut off OD <0.5. (B) ODs of the anti-PF4/heparin IgG EIA (antigen assay) of 4 VITT patients with persistently high ODs over time of undiluted and diluted serial samples. The OD of the anti-PF4/heparin IgG EIA remained high with values of >2.0 over 11 weeks. After dilution of the samples (1:4000 in patients 1-3; 1:2000 in patient 4), ODs again declined, showing that the persistently high OD values were caused by the low dynamic range of the EIA at high antibody concentrations. The dotted line (...) shows the cutoff OD <0.5.
Anti-PF4 IgG antibody levels as measured by optical density (OD) in the anti-PF4/heparin IgG EIA also declined in most patients. At the time of diagnosis, 59 of 65 patients showed an OD >2. The median OD values at the time of the first and last available blood sample decreased by 51.3% (median OD first measurement, 3.12; median OD last measurement, 1.52; signed-rank test P < .0001). The proportion of different anti-PF4/heparin IgG EIA OD thresholds over time is shown in supplemental Figure 1A-D. The anti-PF4/heparin IgG EIA, at the time of the first negative platelet activation assay, was median OD 1.53 with a wide range of OD (0.20-3.00). Considering the individual differences, it is difficult to define a cutoff for the OD, at which platelet-activating antibodies are no longer expected.
However, seroreversion to a negative EIA result (<0.5 OD units) was seen in only 14 patients within the follow-up period (Figure 1A). The temporal decline of anti-PF4 IgG antibodies in VITT is similar to the antibody dynamics in HIT,13 although the VITT antibodies seem to persist considerably longer. Antibody levels in VITT patients are unusually high during acute VITT.6,7 This could help explain why the antibodies remain detectable by the EIA test longer than is usually seen in HIT.
We observed 5 patients with persistently high anti-PF4/heparin EIA ODs. They also showed a substantial decline in antibody levels when sera were assessed at increasing dilutions, exemplified for 4 patients in Figure 1B. This indicates that these antibodies decline in titer but from such high levels that they are still reacting strongly positive in the laboratory assays. All 5 patients received therapeutic dose anticoagulation since VITT occurred. Among them, 2 experienced recurrent episodes of thrombocytopenia, including in 1 patient associated with a new deep vein thrombosis; the other 3 patients remained asymptomatic.
VITT patients tolerate a second vaccination shot with an mRNA vaccine
Twenty-nine patients received a second vaccine shot with the mRNA vaccine Comirnaty (n = 26; BioNTech/Pfizer) or Moderna (n = 3), administered at a median time of 16 weeks (range, 6-31 weeks) after the first shot with ChAdOx1 nCoV-19. Twenty-two of them still received therapeutic dose and 1 prophylactic dose anticoagulation. None of them developed symptomatic new thrombotic complications. In 20/22 patients in whom the platelet count was monitored after vaccination, platelet counts remained stable. Two showed a decrease of 25% to 27% (211 to 158 Gpt/L and 153 to 111 Gpt/L), but both showed decreasing anti-PF4 IgG EIA OD values and no recurrence of platelet-activating antibodies. In 15 patients, a negative platelet activation assay was documented before the second vaccination shot, while in 10 patients, it was still positive. In 3 patients, only the initial sample of VITT diagnosis was available before the second vaccination shot. For 23/29 patients, the anti-PF4/heparin IgG EIA ODs before the second vaccination ranged between 0.5 and 3.3. In 28/29 patients, blood samples were available after the second vaccination (median, 13 days after vaccination; range, 5-79 days). Only 1 patient showed a slight increase in OD of the anti-PF4/heparin IgG EIA (2.47 to 2.82) after the second vaccination (Figure 2).
Optical densities of the anti-PF4/heparin IgG EIA (antigen assay) before and after second vaccination shot against COVID-19. Of 28/29 patients with the second vaccination against COVID-19, serum samples before and after the second vaccination shot were available (median time, 13 days after second vaccination [range, 5-79 days]). The optical density of the anti-PF4/heparin IgG EIA decreased in 21 of the patients, while in 6 patients the OD did not increase beyond the interassay variability (±0.1 OD), overall P = .00043. Only 1 patient showed a slight increase in OD of the anti-PF4/heparin IgG EIA (2.47 to 2.82) after the second vaccination.
Optical densities of the anti-PF4/heparin IgG EIA (antigen assay) before and after second vaccination shot against COVID-19. Of 28/29 patients with the second vaccination against COVID-19, serum samples before and after the second vaccination shot were available (median time, 13 days after second vaccination [range, 5-79 days]). The optical density of the anti-PF4/heparin IgG EIA decreased in 21 of the patients, while in 6 patients the OD did not increase beyond the interassay variability (±0.1 OD), overall P = .00043. Only 1 patient showed a slight increase in OD of the anti-PF4/heparin IgG EIA (2.47 to 2.82) after the second vaccination.
Our observations indicate that mRNA vaccines are very well tolerated regardless of whether PF4-dependent platelet-activating antibodies are still circulating or not. This is highly relevant for patients in countries with no available assays for functional testing of VITT antibodies. In addition, this is very strong in vivo evidence that the mRNA vaccines do not contain or induce the cofactor required for anti-PF4 antibody–mediated prothrombotic activation of platelets. It remains unresolved whether reexposure with an adenovirus vector–based vaccine in patients after VITT is also possible.
Acknowledgments
The authors thank the staff of the transfusion medicine platelet laboratory for their excellent technical support: Ulrike Strobel, Carmen Freyer, Ricarda Raschke, Ines Warnig, Jessica Fuhrmann, Katrin Stein, and Nicole Lembke. The authors are grateful to all the patients and more than 110 treating physicians (hospital and family physicians) who sent blood samples for follow-up and provided us with clinical information.
The study was supported by Deutsche Forschungsgemeinschaft (German Research Foundation) grant 374031971-TRR240. L.S. is supported within the Gerhard-Domagk-Research-Program by the University Medicine Greifswald.
Some of the results reported were obtained in a study conducted by Universitätsmedizin Greifswald under service contract No. EMA/2021/17/TDA. The views expressed are the personal views of the authors and may not be understood or quoted as being made on behalf of or reflecting the position of the European Medicines Agency or one of its Committees or Working Parties.
Authorship
Contribution: L.S. performed the study and took care of patients; T.T. and A. Greinacher developed the concept, analyzed data, and took care of patients and laboratory studies; S.E.S. summarized patient data; L.K. performed the biostatistical analyses, created the figures, and helped write the manuscript; A. Günther, K.S., and T.H. took care of patients and laboratory assays; L.S., A. Greinacher, T.T., and L.K. wrote the manuscript; and all authors have critically revised and approved the final version of the manuscript. A. Greinacher, T.T., L.S., L.K., and S.E.S. have accessed and verified the underlying data.
Conflict-of-interest disclosure: A. Greinacher reports grants and nonfinancial support from Aspen, Boehringer Ingelheim, MSD, Bristol Myers Squibb (BMS), Paringenix, Bayer Healthcare, Gore Inc., Rovi, Sagent, Biomarin/Prosensa, personal fees from Aspen, Boehringer Ingelheim, MSD, Macopharma, BMS, Chromatec, Instrumentation Laboratory, nonfinancial support from Boehringer Ingelheim, Portola, Ergomed, GTH e.V. outside the submitted work. T.T. reports personal fees and other from Bristol Myers Squibb, personal fees and other from Pfizer, personal fees from Bayer, personal fees and other from Chugai Pharma, other from Novo Nordisk, personal fees from Novartis, other from Daiichi Sankyo, outside the submitted work. L.S. receives a young investigator grant from the medical faculty of the Universitätsmedizin Greifswald. A. Günther reports speakers’ honoraria from Bayer Vital GmbH, Bristol Myers Squibb, Daiichi Sankyo, a research grant from IPSEN outside the submitted work. The remaining authors declare no competing financial interests.
Correspondence: Andreas Greinacher, Institut für Immunologie und Transfusionsmedizin, Universitätsmedizin Greifswald, Sauerbruchstraße, D-17489 Greifswald, Germany; e-mail: andreas.greinacher@med.uni-greifswald.de.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.

![Optical densities of the anti-PF4/heparin IgG EIA (antigen assay) before and after second vaccination shot against COVID-19. Of 28/29 patients with the second vaccination against COVID-19, serum samples before and after the second vaccination shot were available (median time, 13 days after second vaccination [range, 5-79 days]). The optical density of the anti-PF4/heparin IgG EIA decreased in 21 of the patients, while in 6 patients the OD did not increase beyond the interassay variability (±0.1 OD), overall P = .00043. Only 1 patient showed a slight increase in OD of the anti-PF4/heparin IgG EIA (2.47 to 2.82) after the second vaccination.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/139/12/10.1182_blood.2021014214/3/m_bloodbld2021014214f2.png?Expires=1769115615&Signature=VMPKBTEWCAysGTX5nLDF6-0iQrxGjJx26IMljBurW7c9BjcJMxazJbU4c4ZzuHmo5V9kiSLEf~Y7tVgs7dEPMdZWA3J1xy3V7V0fjfwlnZRpRfw9J~iZt5XlHhzoKU7X3QhTPEwLSFILsPWLWQCFRarxh0DWqr3ct~MUqp3A0oRmAw9ZJqo-lW-tmwEfqSwpaxPPToHgx27BGDrb5PoQ6VL831FbLo7MrcR42XLIqdhA7kI1aTEDsRDzevfkbyLV2vYqC98WlvYC5cpsMydADLpmi0uRUjiRYEXbNKxtnk~HXtqkGX0iaCrWPcRcStSBvdqzTRp0umdnE-Pgjg8ZfQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal