Issue Archive
Table of Contents
BLOOD COMMENTARIES
PLENARY PAPER
Ibrutinib, obinutuzumab, and venetoclax in relapsed and untreated patients with mantle cell lymphoma: a phase 1/2 trial
Clinical Trials & Observations
In a Plenary Paper, Le Gouill et al present results of a phase 1/2 trial of ibrutinib, obinutuzumab, and venetoclax in mantle cell lymphoma. With a median follow-up of 14 months, they demonstrate excellent outcomes with triple therapy in untreated, relapsed, and high-risk patients. Many patients achieved minimal residual disease–negative disease, including 100% of treatment-naïve patients. Longer follow-up is required to confirm whether this predicts for long-term remission.
BLOOD SPOTLIGHT
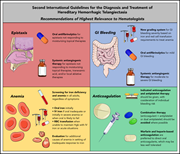
Hereditary hemorrhagic telangiectasia: systemic therapies, guidelines, and an evolving standard of care
In a Blood Spotlight, Al-Samkari reviews the current recommendations from the Second International Guidelines for the Diagnosis and Treatment of Hereditary Hemorrhagic Telangiectasia.
CLINICAL TRIALS AND OBSERVATIONS
A randomized phase 2 trial of pomalidomide in subjects failing prior therapy for chronic graft-versus-host disease
Clinical Trials & Observations
Curtis and colleagues present results of a phase 1/2 trial of pomalidomide for steroid-refractory graft-versus-host disease (GVHD). They demonstrate promising results with overall partial response rates of 47% by intention to treat and 67% among patients treated for 6 months. This study establishes the basis for a phase 3 trial of low-dose pomalidomide (0.5 mg/day) for treatment of refractory GVHD.
HEMATOPOIESIS AND STEM CELLS
UTX maintains the functional integrity of the murine hematopoietic system by globally regulating aging-associated genes
IMMUNOBIOLOGY AND IMMUNOTHERAPY
Permissive HLA-DPB1 mismatches in HCT depend on immunopeptidome divergence and editing by HLA-DM
Brief Report
LYMPHOID NEOPLASIA
Long-term results of PET-guided radiation in patients with advanced-stage diffuse large B-cell lymphoma treated with R-CHOP
Clinical Trials & Observations
The role of consolidative radiotherapy (RT) following R-CHOP therapy for advanced diffuse large B-cell lymphoma (DLBCL) remains controversial. Freeman et al report on a nonrandomized end-of-therapy positron emission tomography–computed tomography (PET-CT)-guided approach to consolidative RT in which RT was excluded for PET-negative patients. Outcomes for PET-negative patients and irradiated PET-positive patients were comparable, suggesting that PET-CT may guide therapy for selective administration of consolidative RT.
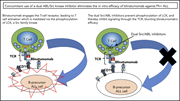
Concomitant use of a dual Src/ABL kinase inhibitor eliminates the in vitro efficacy of blinatumomab against Ph+ ALL
Brief Report
Blinatumomab is approved for treatment of relapsed/refractory acute lymphoblastic leukemia (ALL), but combined therapy with tyrosine kinase inhibitors (TKIs) have been shown in small studies to improve response. Since the cytotoxicity of blinatumomab is mediated by T-cell receptor signaling, Leonard et al studied the impact of third generation Src/ABL kinase inhibitors (ponatinib and dasatinib) on T-cell responses. They demonstrate in vitro inhibition of T cells and loss of blinatumomab toxicity. This potential inhibition injects caution into combinations using these TKIs, but it must be squared with the efficacy of these combined agents in humans in small published studies.
MYELOID NEOPLASIA
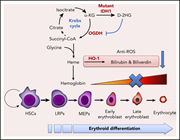
IDH1 mutation contributes to myeloid dysplasia in mice by disturbing heme biosynthesis and erythropoiesis
Gu et al demonstrate that IDH1 mutations contribute to dyserythropoiesis by disrupting heme biosynthetic pathways.The aberrant metabolite D-2-hydroxyglutarate decreases succinyl coenzyme A, which attenuates heme biosynthesis, decreases heme oxygenase 1 expression and leads to increased reactive oxygen species that induce apoptosis in erythroid progenitors.
PLATELETS AND THROMBOPOIESIS
Transcriptional profile of platelets and iPSC-derived megakaryocytes from whole-genome and RNA sequencing
The authors performed RNA sequencing on induced pluripotent stem cell (iPSC)–derived megakaryocytes and matched platelet samples to delineate shared and unshared cis-expression quantitative trait loci (eQTLs) that potentially influence platelet biology. This data set may inform future studies of genetic influences on platelet function contributing to thrombosis, hemostasis, and inflammation.
THROMBOSIS AND HEMOSTASIS
Cost effectiveness of caplacizumab in acquired thrombotic thrombocytopenic purpura
Clinical Trials & Observations
The authors performed a cost-effectiveness analysis of adding caplacizumab to the standard of care for acquired thrombotic thrombocytopenic purpura (TTP). They conclude that uniform addition of caplacizumab is not cost-effective. Whether the cost is balanced by improved outcomes in certain subgroups of patients with TTP will require longer-term follow-up data.
TRANSPLANTATION
A validated pediatric disease risk index for allogeneic hematopoietic cell transplantation
CME
In this month’s CME article, the authors stratify risk of allogeneic hematopoietic stem cell transplantation for children under the age of 18 years with acute myeloid (AML) or lymphoblastic (ALL) leukemia based on over 2500 patients divided into training and validation cohorts. They define 4 AML risk groups and 3 ALL risk groups, based on age, disease status, and cytogenetic risk, that facilitate prediction of leukemia-free survival.
LETTER TO BLOOD
Epigenetic loss of m1A RNA demethylase ALKBH3 in Hodgkin lymphoma targets collagen, conferring poor clinical outcome
BLOOD WORK
CONTINUING MEDICAL EDUCATION (CME) QUESTIONS
-
Cover Image
Cover Image
![issue cover]()
D-2-hydroxyglutarate (D-2HG) impedes the erythroid differentiation of K562 leukemia cells by inhibiting enzymatic activity of oxoglutarate dehydrogenase (OGDH) and therefore reducing heme biosynthesis. See the article by Gu et al on page 945.
- PDF Icon Front MatterFront Matter
- PDF Icon Table of ContentsTable of Contents
- PDF Icon Back MatterBack Matter
- PDF Icon Editorial BoardEditorial Board
Advertisement intended for health care professionals
Email alerts
Advertisement intended for health care professionals











Building on BTK inhibition in MCL
Clinical Trials & Observations