In this issue of Blood, Curtis et al present the results of a randomized phase 2 trial demonstrating activity and safety of pomalidomide for advanced chronic graft-versus-host disease (cGVHD) with fibrotic manifestations involving joint, fascia, and skin. This article is of a broad interest to hematologists and hematopoietic cell transplantation (HCT) providers, since it addresses an unmet medical need for patients with glucocorticoid-refractory cGVHD with advanced fibrotic manifestations.1
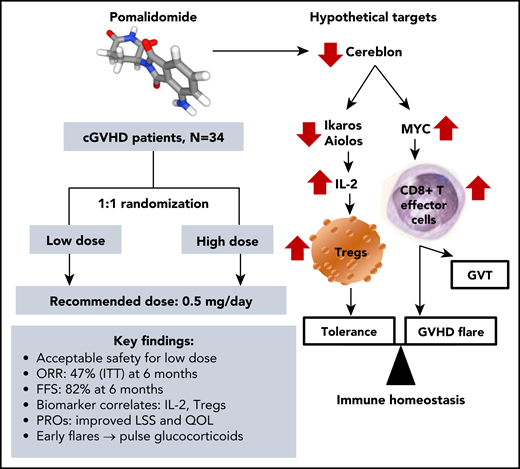
Pomalidomide therapy for cGVHD: trial highlights and immunomodulatory targets. FFS, failure-free survival; GVT, graft-versus-tumor; IL-2, interleukin-2; ITT, intent-to-treat; LLS, Lee symptom scale; ORR, overall response rate; PROs, patient-reported outcomes; QOL, quality of life; Tregs, regulatory T-cells.
Pomalidomide therapy for cGVHD: trial highlights and immunomodulatory targets. FFS, failure-free survival; GVT, graft-versus-tumor; IL-2, interleukin-2; ITT, intent-to-treat; LLS, Lee symptom scale; ORR, overall response rate; PROs, patient-reported outcomes; QOL, quality of life; Tregs, regulatory T-cells.
The high potency of pomalidomide is paired with more favorable toxicity profile compared with its structurally related immunomodulatory drugs (IMiDs). While thalidomide demonstrated variable efficacy in multiple trials of cGVHD,2 it currently has a very limited use in advanced cGVHD, as its potentially effective dose of ≥200 mg/day is often associated with excessive neurologic, gastrointestinal, and hematologic toxicities. A broader use of lenalidomide in GVHD was halted by the risks of myelosuppression and GVHD propagation. Notably, lenalidomide maintenance after allogeneic HCT in patients with multiple myeloma increased incidence of acute GVHD in pivotal HOVON 76 and 07-REV trials.3,4 The multicenter study by Curtis et al extends findings from the prior early-phase trial of pomalidomide in a smaller group of allograft recipients with glucocorticoid-refractory moderate-to-severe cGVHD.5 Despite promising early efficacy, pomalidomide was poorly tolerated at the dose of 3 mg/day in that trial.5 The phase 2 trial by Curtis et al has determined oral pomalidomide 0.5 mg/day as the optimal therapeutic dose for future use in sclerotic cGVHD (see figure).
Patients with extensive sclerotic cGVHD are often refractory to available therapies and have poor overall survival.6 Curtis et al demonstrate that pomalidomide benefits fibrotic phenotypes of cGVHD. Imatinib and rituximab were compared in a randomized phase 2 trial of cGVHD patients with cutaneous sclerosis, but both led to suboptimal significant clinical responses at 6 months (26% and 27%, respectively).7 In this study by Curtis et al, the overall response rate to pomalidomide was 47% in an intent-to-treat analysis and 67% among all evaluable patients at 6 months. Significant improvements in joint/fascia National Institutes of Health scores and skin involvement were achieved in patients with a median of 5 prior lines of therapy and 5 organs affected by cGVHD. Such heavily pretreated patients include a representative real-world sample of the cGVHD population impacted by substantial disability, morbidity, and impaired quality of life.
Failure-free survival has emerged as an important composite study end point of treatment change, nonrelapse mortality, and recurrent malignancy, used across clinical trials of cGVHD. Curtis et al report a noteworthy FFS of 82% at 6 months which compares favorably to the benchmark of 56% at 6 months after second line therapy of cGVHD.8 According to Curtis et al, treatment failures (1-FFS) were largely attributed to the change of therapy (48%) yet very limited nonrelapse mortality (3%). The positive results of this trial are further strengthened by clinically meaningful improvements in Lee symptom scale and other patient-reported outcomes.1
Pomalidomide has a potent stimulatory effect leading to overproduction of IL-2 among multiple other cytokines.2 Increased IL-2 levels observed in this trial are consistent with an established effect of pomalidomide, and the concomitant increase in the Treg fraction is likely secondary to the IL-2 burst. The complex immunomodulatory effects of pomalidomide may vary across the key phases of cGVHD pathobiology involving tissue damage, adaptive immunity, and end-organ fibrosis. Recent advances in cGVHD biology have underscored the critical role of Treg cells in restoring immune tolerance and balancing inflammatory responses related to T helper type 17/type 17 CD8+ T cell upregulation as the cornerstones of cGVHD pathogenesis.9 Thus, one of the mechanisms of pomalidomide suppression of cGVHD appears to be IL-2 upregulation and expansion of Treg cells (see figure). Obviously, pomalidomide does not consistently induce stable tolerance, because a fraction of patients experienced cGVHD recurrence when the drug was stopped.1
Curtis et al observed no relapsed malignancy with the use of pomalidomide within 2 years of follow-up. While cGVHD has been previously associated with less relapse after HCT, this observation suggests a potential role of pomalidomide in sparing or even augmenting the graft-versus-tumor effect. By targeting cereblon, IMiDs can generate metabolically hyperactive human CD8+ T cells with effector phenotype and thereby enhance antitumor activity.10 In mice, the antitumor effect of cereblon modulating compounds was also accompanied by accelerated GVHD.10 Perhaps such a “double-edged sword” effect of pomalidomide might explain early flares (within 6 months) of cGVHD among ∼18% of the trial participants as reported by Curtis et al.
Altogether, this novel, well-designed, well-conducted trial provides compelling evidence for adding pomalidomide to current treatment armamentarium of glucocorticoid-refractory sclerotic cGVHD. These data also support using pomalidomide for future trials incorporating experimental and/or the standard of care comparator arms. As novel therapies for cGVHD continue to emerge, it becomes increasingly important to tailor each one of those to an individual patient based on clinical cGVHD phenotypes, pathophysiologic mechanisms (eg, inflammatory and fibrotic), and underlying immune dysregulation. Besides facilitating personalized therapy for cGVHD, such insights will also inform rationale drug combinations as well as an understanding of which patients are expected to derive most clinical benefit from IMiDs, Janus kinase, Bruton tyrosine kinase inhibitors, or numerous other promising therapies being developed for cGVHD.
Conflict-of-interest disclosure: A.L. has received consultancy payment from EUSA Pharma and owns limited equities of Bristol Myers Squibb.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal