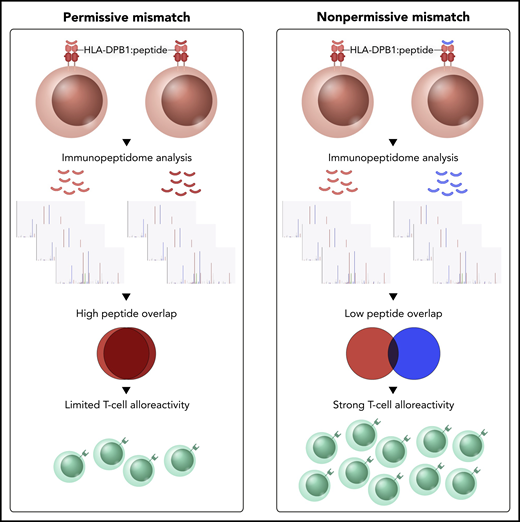
In this issue of Blood, Meurer et al1 report on the mechanistic underpinning of permissive HLA-DPB1 mismatch using mass spectrometry–based analysis of the HLA-DP immunopeptidome, T-cell receptor β (TCRβ) clonotype sequencing, and exploration of HLA-DM–mediated peptide editing. Permissive HLA-DPB1 mismatches show greater overlap in their HLA-presented peptide repertoire compared with their nonpermissive counterparts. This results in both a lower frequency and diversity of alloreactive TCRβ clonotypes (see figure), which could be reversed by silencing of the peptide editor HLA-DM.
Schematic illustration of the immunopeptide-based mechanism of permissive and nonpermissive HLA mismatch. Permissive HLA-DPB1 mismatches show greater overlap in their HLA-presented peptide repertoire compared with their nonpermissive counterparts, resulting in lower T-cell alloreactivity.
Schematic illustration of the immunopeptide-based mechanism of permissive and nonpermissive HLA mismatch. Permissive HLA-DPB1 mismatches show greater overlap in their HLA-presented peptide repertoire compared with their nonpermissive counterparts, resulting in lower T-cell alloreactivity.
The graft-versus-leukemia (GVL) effect following allogeneic stem cell transplantation still represents one of the most compelling examples of cancer immunotherapy. Since the first transplantation for leukemia, reported by E. Donnall Thomas in 1957, the optimal equilibrium of T-cell alloreactivity sufficient for the GVL effect, but not for severe graft-versus-host disease (GVHD), has been the fundamental goal of allogeneic hematopoietic stem cell therapy (HCT). The discovery and delineation of HLAs enabled matching between donors and recipients but also allowed for the identification of the peptide antigens presented via these molecules on the cell surface called the immunopeptidome. In recent years, in-depth immunopeptidome analyses of various cancer entities, including hematological malignancies, enabled the identification and characterization of the targets of anticancer T-cell responses and paved the way for the understanding, optimization, and new development of T-cell–based immunotherapies, including HCT.2 In contrast to HLA-matched sibling donors, where minor histocompatibility antigens almost exclusively represent the targets of T-cell alloreactivity, most HLA-matched unrelated donors (UDs) additionally present mismatches for the HLA-DP antigens eliciting direct alloreactive T-cell responses with important implications for both GVHD and GVL. Increasing evidence suggested that donor-recipient disparities for HLA-DPB1 can be of clinical importance.3 HLA-DPB1 mismatch was reported to elicit a wide range of alloreactive T-cell responses associated with not only an increased risk of acute GVHD4 but also a reduced risk of leukemic relapse due to the GVL.5 As HLA-DPB1 mismatch is observed in >80% of UD-recipient pairs, the definition of permissive HLA-DPB1 mismatches (ie, HLA-DPB1 combinations eliciting limited T-cell alloreactivity with a reduced risk of GVHD but maintaining the clinical efficacy of GVL) represents a major challenge and opportunity in HLA-matched UD-HCT. The differentiation between permissive and nonpermissive was achieved by functional matching for T-cell epitope (TCE) groups displaying cross-recognition between different HLA-DPB1 alleles.6 Permissive HLA-DPB1 mismatches were shown to significantly associated with a lower risk of mortality and relapse after HCT, prompting the inclusion of the HLA-DPB1 TCE algorithm into international donor selection guidelines.7 However, so far, the detailed mechanisms underlying permissive and nonpermissive HLA-DPB1 mismatch have not been clearly delineated. Based on their own preliminary work suggesting a role of the immunopeptidome presented by different HLA-DPB1 allotypes on TCE permissiveness,8 Meurer's group investigated the diversity of peptide repertoires in a model of specific permissive vs nonpermissive HLA-DP allelic variant combinations. Comparing different HLA-DP allotypes from 3 TCE groups on different cell lines, they detected a significantly higher overlap between the immunopeptidomes of permissive vs nonpermissive allotype combinations, which resulted in lower frequency and diversity of alloreactive TCRβ clonotypes in healthy donors and patients after HCT. Thus, mass spectrometry–based immunopeptidome and TCRβ clonotype sequencing analyses might contribute to the characterization of permissiveness in currently debated HLA-DPB1 mismatch combinations and also for other HLA loci. Furthermore, it might complement prediction algorithms that relate HLA expression levels with the risk of GVHD after UD-HCT.9
Strikingly, further analysis on the mechanistical basis of immunopeptidome diversity in HLA-DP permissive and nonpermissive mismatch identified a central role of the peptide editor HLA-DM. HLA-DM regulates the peptide loading from self-antigens or foreign antigens on HLA class II molecules by catalyzing the removal of the residual HLA class II cleaved invariant chain peptide as well as other weak-binding peptides and their replacement by strong-binding HLA ligands. By preventing the presentation of weak-binding peptides, HLA-DM guides the T-cell response to “immunodominant” regions of antigens, which in the case of self-proteins promotes elimination of potentially autoreactive T cells.10 Meurer et al showed that the absence of HLA-DM led to an increase in the number and abundance of HLA-DP peptides with a significant increase in T-cell alloreactivity to permissive HLA-DP allotype combinations in healthy donors as well as in patients after HCT. These data underscore the role of HLA-DM peptide editing in the context of UD-HCT and provide, alongside novel immunopeptidome-based algorithms for donor selection, major implications for future treatment approaches. Further validation and expansion of these results in primary leukemia samples is warranted and might enable the characterization of HLA-DM–edited high-affinity peptides serving as target structures for T-cell–based immunotherapy approaches and even guide pharmacological or gene-editing–based immunopeptidome modulation in HCT.
Conflict-of-interest disclosure: The author declares no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal