Key Points
Dual Src/ABL inhibitors dasatinib and ponatinib inhibited blinatumomab-induced T-cell proliferation in vitro at nanomolar concentrations.
Potential immunomodulatory effects of targeted therapies should be taken into consideration before they are combined with immunotherapies.
Abstract
Blinatumomab is currently approved for use as a single agent in relapsed and refractory acute lymphoblastic leukemia (ALL). Cytotoxicity is mediated via signaling through the T-cell receptor (TCR). There is now much interest in combining blinatumomab with targeted therapies, particularly in Philadelphia chromosome–positive ALL (Ph+ ALL). However, some second- and third-generation ABL inhibitors also potently inhibit Src family kinases that are important in TCR signaling. We combined ABL inhibitors and dual Src/ABL inhibitors with blinatumomab in vitro from both healthy donor samples and primary samples from patients with Ph+ ALL. Blinatumomab alone led to both T-cell proliferation and elimination of target CD19+ cells and enhanced production of interferon-γ (IFN-γ). The addition of the ABL inhibitors imatinib or nilotinib to blinatumomab did not inhibit T-cell proliferation or IFN-γ production. However, the addition of dasatinib or ponatinib inhibited T-cell proliferation and IFN-γ production. Importantly, there was no loss of CD19+ cells treated with blinatumomab plus dasatinib or ponatinib in healthy samples or samples with a resistant ABL T315I mutation by dasatinib in combination with blinatumomab. These in vitro findings bring pause to the excitement of combination therapies, highlighting the importance of maintaining T-cell function with targeted therapies.
Introduction
Although great strides have been made with systemic cytotoxic therapy for the treatment of acute lymphoblastic leukemia (ALL), there remain subsets of patients who continue to die from their disease. For patients harboring the BCR/ABL fusion (Philadelphia chromosome–positive ALL [Ph+ ALL]), the addition of ABL and Src/ABL inhibitors to their therapies have led to great improvements in patient outcomes.1-4 However, relapses continue to make effective therapies a great need for this patient population. Blinatumomab, a bi-specific T-cell engager, was recently approved for the treatment of relapsed B-ALL or persistent minimal residual disease after treatment, including patients with Ph+ ALL.5,6
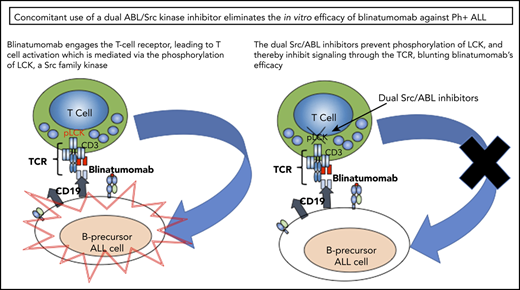
Combining a targeted inhibitor with blinatumomab is a reasonable next step, and preliminary clinical findings are intriguing.7 However, the second- and third-generation ABL inhibitors dasatinib and ponatinib are also known to inhibit Src family kinases,8-11 whereas the second-generation ABL inhibitor nilotinib is not a potent Src inhibitor.12,13 T-cell receptor (TCR) signaling, critical for T-cell activation, is known to be dependent on Src family kinase activity through LCK.14-16 This would suggest that these inhibitors may impact the efficacy of immunotherapies reliant on native T-cell function. With this concern, we sought to investigate the effects of the ABL specific and the dual Src/ABL inhibitors on blinatumomab’s effects on T-cell proliferation, activation through interferon-γ (IFN-γ) expression, and cytotoxicity through B-cell clearance using both healthy donor peripheral blood mononuclear cells and primary patient samples from patients with Ph+ ALL.
Study design
Peripheral blood mononuclear cells (PBMCs) were obtained from 5 healthy volunteers and from 5 patients with Ph+ ALL after informed consent was obtained through an institutional review board–approved protocol. The Ph+ ALL samples included 3 samples from newly diagnosed patients and 2 from patients with relapsed Ph+ ALL harboring a T315I mutation.
PBMCs were labeled with CellTrace Violet (ThermoFisher, Waltham, MA) and cultured for 5 days with no stimulation, 10 nM blinatumomab alone, or 10 nM blinatumomab in combination with imatinib, dasatinib, ponatinib, or nilotinib at concentrations from 5 to 500 nM. To test the effect of intermittent exposure to dasatinib, PBMCs from 3 healthy donors were labeled with CellTrace Violet and cultured with 10 nM blinatumomab and 10 nM dasatinib for 2 hours, and media were removed and replaced with media containing 10 nM blinatumomab only for 22 hours. This was repeated for 5 days.
Immunophenotyping was performed using multiparameter flow cytometry for the following cell surface markers: CD45 (HI30), CD3 (UCHT1), CD4 (RPA-T4), CD8 (RPA-T8), and CD19 (HIB19). Quantification of B cells was assessed by comparing the numbers of CD19+/CD3− cells in untreated samples with those that had been treated with blinatumomab +/− the tyrosine kinase inhibitors (TKIs). Division of T cells was measured by CellTrace Violet dilution.
Media from each sample after treatment as above were assessed for IFN-γ using LEGENDplex Human Th Cytokine Panel (BioLegend, San Diego, CA). Comparison between samples was assessed by Student t test using Graph Pad Prism.
Jurkat cells were cultured with CD3 and CD28 alone or with increasing concentrations of imatinib, nilotinib, dasatinib, and ponatinib at concentrations ranging from 0.5 to 500 nM for 4 hours or cultured with CD3 and CD28 alone for 2 hours followed by the addition of TKI for 2 hours. Cells were harvested, and cell lysate was separated by standard sodium dodecyl sulfate-polyacrylamide gel electrophoresis, transferred onto polyvinylidene fluoride, and immunoblotted with anti–P-LCK (Tyr394 Phospho-LCK) and anti-Src (TYR416 Phospho-Src family). Quantification of immunoblots was performed using ImageJ (National Institutes of Health, Bethesda, MD).
Results and discussion
Treatment with Src/ABL inhibitors decreases efficacy of blinatumomab on CD3+ T-cell proliferation and elimination of CD19+ B cells in vitro
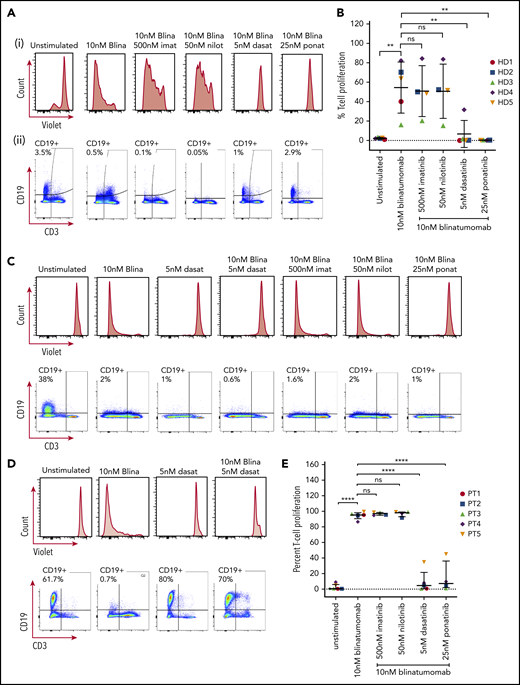
PBMCs isolated from healthy donors showed T-cell proliferation after 5 days with 10 nM blinatumomab (Figure 1A-B). In the presence of imatinib and nilotinib, there continued to be T-cell proliferation. In contrast, no T-cell proliferation was found if the cells were treated with either dasatinib or ponatinib. This finding was further validated by the lack of CD19+ B-cell clearance in the presence of dasatinib and ponatinib (Figure 1A-B).
T-cell proliferation and CD19+B-cell clearance. (A) PBMC response to treatment from a representative healthy donor. Cells from healthy donors were cultured for 5 days alone or with 10 nM blinatumomab with or without 500 nM imatinib, 50 nM nilotinib, 5 nM dasatinib, or 25 nM ponatinib. Cells were then assessed by multiparametric flow cytometry gated on CD45+ cells. (i) Histograms of CD3+ cells assessed for retention of CellTrace Violet. Loss of intensity is a marker of cell proliferation. (ii) CD19+ expression and CD3+ expression from these samples. Percentage of CD19+ cells is shown in the upper left quadrant of each histogram. (B) Combined results of T-cell proliferation from 5 healthy donor samples. Percent of T-cell proliferation was quantified by measuring the percent of T cells diluting CellTrace Violet. Comparisons were performed by Student t test (**P < .01). Each shape represents an individual healthy donor. (C) PBMCs from a representative newly diagnosed patient with Ph+ ALL. The sample was incubated for 5 days in the presence of drugs as described above and assessed for T-cell proliferation by CellTrace Violet retention and expression of CD19 and CD3. (D) Response of PBMCs from a patient with relapsed Ph+ ALL with a T315I mutation. (E) Combined percent T-cell proliferation from 5 Ph+ ALL samples (3 primary Ph+ ALL and 2 relapsed Ph+ ALL T315I). Each column was compared by Student t test (****P < .0001). Each shape represents an individual patient sample.
T-cell proliferation and CD19+B-cell clearance. (A) PBMC response to treatment from a representative healthy donor. Cells from healthy donors were cultured for 5 days alone or with 10 nM blinatumomab with or without 500 nM imatinib, 50 nM nilotinib, 5 nM dasatinib, or 25 nM ponatinib. Cells were then assessed by multiparametric flow cytometry gated on CD45+ cells. (i) Histograms of CD3+ cells assessed for retention of CellTrace Violet. Loss of intensity is a marker of cell proliferation. (ii) CD19+ expression and CD3+ expression from these samples. Percentage of CD19+ cells is shown in the upper left quadrant of each histogram. (B) Combined results of T-cell proliferation from 5 healthy donor samples. Percent of T-cell proliferation was quantified by measuring the percent of T cells diluting CellTrace Violet. Comparisons were performed by Student t test (**P < .01). Each shape represents an individual healthy donor. (C) PBMCs from a representative newly diagnosed patient with Ph+ ALL. The sample was incubated for 5 days in the presence of drugs as described above and assessed for T-cell proliferation by CellTrace Violet retention and expression of CD19 and CD3. (D) Response of PBMCs from a patient with relapsed Ph+ ALL with a T315I mutation. (E) Combined percent T-cell proliferation from 5 Ph+ ALL samples (3 primary Ph+ ALL and 2 relapsed Ph+ ALL T315I). Each column was compared by Student t test (****P < .0001). Each shape represents an individual patient sample.
In Ph+ ALL samples from both newly diagnosed patients and those with an ABL T315I mutation, again we could see T-cell proliferation and clearance of CD19+ B cells with the treatment of blinatumomab (Figure 1C-E). As expected, the addition of treatment with imatinib or nilotinib did not decrease T-cell proliferation or prevent the clearance of CD19+ B cells. In contrast, concentrations as low as 5 nM for dasatinib and 25 nM for ponatinib appeared to inhibit T-cell proliferation. In newly diagnosed patient samples, blinatumomab with dasatinib and ponatinib continued to show clearance of the CD19+ B cells despite inhibition of T-cell proliferation, presumably because of their activity against the ABL kinase (Figure 1C). Strikingly, in the patients with the T315I mutation, the addition of dasatinib not only inhibited T-cell proliferation but also prevented clearance of the CD19+ B cells (Figure 1D). Dasatinib alone, as expected, had no effect on CD19+ cells in the patient with the T315I mutation. Taken together, this suggests that the anti-ABL activity of the TKIs rather than the immune effects of blinatumomab in culture drive the primary activity of the combination of blinatumomab with dasatinib or ponatinib in the patient samples.
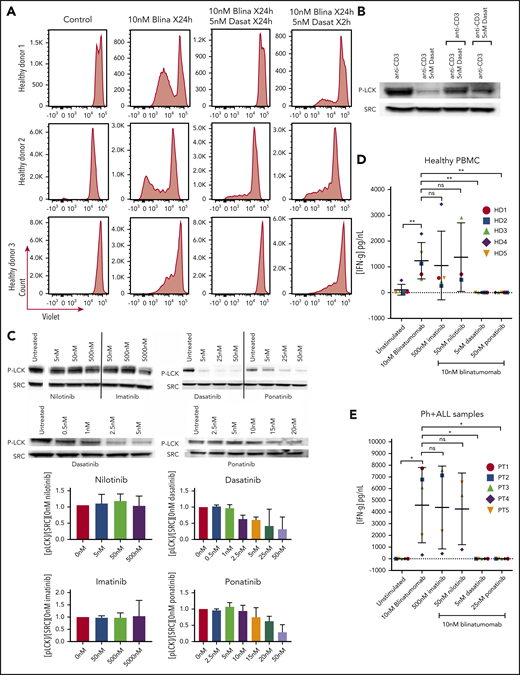
Our in vitro TKI exposure clearly does not reflectin vivo drug concentrations. In patients with chronic myeloid leukemia treated with dasatinib, plasma concentrations of dasatinib fell to below 5 nM within 4 to 8 hours of administration.17 Other pharmacokinetic data have well described that imatinib and nilotinib act by continuous inhibition, whereas dasatinib has intermittent exposure. In vitro, we found that dasatinib’s effects on LCK are also rapidly inducible and reversible, and thus based on the pharmacokinetics of dasatinib, blinatumomab’s efficacy may be fully restored when dasatinib levels fall below 5 nM. Given that the half-life of dasatinib (and ponatinib) is between 2 and 4 hours, PBMCs from healthy donors were isolated and cultured with the combination of blinatumomab and dasatinib for 2 hours, and then media were replaced with a blinatumomab-only containing media for 22 hours, which was repeated for 5 days. The removal of dasatinib did allow for T-cell proliferation; however, the response was blunted compared with the samples treated with continuous blinatumomab (Figure 2A).
Assessment of brief exposure, cytokine release, and activation of LCK. (A) PBMC response to brief treatment of dasatinib from 3 healthy donors. Cells from healthy donors were cultured for 5 days alone or with 10 nM blinatumomab with 5 nM dasatinib for 24 hours compared with 2 hours. Cells were then assessed by multiparametric flow cytometry gated for CD45+ cells. Histograms of CD3+ cells were assessed for retention of CellTrace Violet. Loss of intensity is a marker of cell proliferation. (B) Recovery of the T-cell response after dasatinib treatment. Jurkat cells were cultured with anti-CD3 and anti-CD28, or anti-CD3 and anti-CD28 plus dasatinib for 2 hours. After 2 hours, anti-CD3–treated cells were treated with anti-CD3 plus dasatinib, and the CD3 plus dasatinib treated cells were treated with anti-CD3 only. Cell lysate was then isolated, separated, and immunoblotted for phospho-tyr 394 LCK (P-LCK) or total Src. (C) Assessment of LCK phosphorylation by treatment with ABL kinase inhibitors. Jurkat cells were stimulated at baseline with CD3 and CD28 for 4 hours without (untreated) or with nilotinib, imatinib, dasatinib, and ponatinib at the specified concentration. Cell lysate was then isolated, separated, and immunoblotted for phospho-tyr 394 LCK (P-LCK) or total Src. P-LCK blots were quantified compared with total SRC and then normalized to untreated. Histograms below the blots represent the percent inhibition with the standard deviation. (D) IFN-γ expression from PBMCs from healthy donors. Samples were either unstimulated or treated with 10 nM blinatumomab with or without 500 nM imatinib, 50 nM nilotinib, 5 nM dasatinib, or 25 nM ponatinib for 5 days. Media were then harvested and assessed for concentration of IFN-γ (IFN-γ). Comparisons were performed by Student t test (*P < .05, **P < .01). (E) IFN-γ expression from Ph+ ALL samples. Samples were either unstimulated or or treated with 10 nM blinatumomab with or without 500 nM imatinib, 50 nM nilotinib, 5 nM dasatinib or 25 nM ponatinib for 5 days. Media were then harvested and assessed for concentration of IFN-γ. Comparisons were performed bu Student t test (*P < .05, **P < .01).
Assessment of brief exposure, cytokine release, and activation of LCK. (A) PBMC response to brief treatment of dasatinib from 3 healthy donors. Cells from healthy donors were cultured for 5 days alone or with 10 nM blinatumomab with 5 nM dasatinib for 24 hours compared with 2 hours. Cells were then assessed by multiparametric flow cytometry gated for CD45+ cells. Histograms of CD3+ cells were assessed for retention of CellTrace Violet. Loss of intensity is a marker of cell proliferation. (B) Recovery of the T-cell response after dasatinib treatment. Jurkat cells were cultured with anti-CD3 and anti-CD28, or anti-CD3 and anti-CD28 plus dasatinib for 2 hours. After 2 hours, anti-CD3–treated cells were treated with anti-CD3 plus dasatinib, and the CD3 plus dasatinib treated cells were treated with anti-CD3 only. Cell lysate was then isolated, separated, and immunoblotted for phospho-tyr 394 LCK (P-LCK) or total Src. (C) Assessment of LCK phosphorylation by treatment with ABL kinase inhibitors. Jurkat cells were stimulated at baseline with CD3 and CD28 for 4 hours without (untreated) or with nilotinib, imatinib, dasatinib, and ponatinib at the specified concentration. Cell lysate was then isolated, separated, and immunoblotted for phospho-tyr 394 LCK (P-LCK) or total Src. P-LCK blots were quantified compared with total SRC and then normalized to untreated. Histograms below the blots represent the percent inhibition with the standard deviation. (D) IFN-γ expression from PBMCs from healthy donors. Samples were either unstimulated or treated with 10 nM blinatumomab with or without 500 nM imatinib, 50 nM nilotinib, 5 nM dasatinib, or 25 nM ponatinib for 5 days. Media were then harvested and assessed for concentration of IFN-γ (IFN-γ). Comparisons were performed by Student t test (*P < .05, **P < .01). (E) IFN-γ expression from Ph+ ALL samples. Samples were either unstimulated or or treated with 10 nM blinatumomab with or without 500 nM imatinib, 50 nM nilotinib, 5 nM dasatinib or 25 nM ponatinib for 5 days. Media were then harvested and assessed for concentration of IFN-γ. Comparisons were performed bu Student t test (*P < .05, **P < .01).
Treatment with Src/ABL inhibitors decreases IFN-γ expression and phosphorylation of LCK
As blinatumomab is known to transiently induce a cytolytic synapse between the cytotoxic T cell and cells expressing CD19, this activation leads to production of cytokines including IFN-γ.18,19 Treatment of PBMCs with blinatumomab led to a significant increase in production of IFN-γ compared with unstimulated controls in both healthy donor and patient samples (Figure 2D-E). The addition of imatinib or nilotinib did not affect the production of IFN-γ in either healthy PBMCs or Ph+ ALL samples. Again, in contrast, both dasatinib and ponatinib completely suppressed blinatumomab-induced IFN-γ secretion.
As a possible mechanism for the inhibition of T-cell proliferation and IFN-γ production, we assessed intracellular signaling through activation of LCK14 in Jurkat T cells. The addition of dasatinib or ponatinib to the cultures inhibited phosphorylation of LCK with no effect by nilotinib and imatinib (Figure 2C). The phosphorylation of LCK via CD3 can be reversed within 2 hours by the addition of dasatinib, and the inhibition of LCK phosphorylation by dasatinib can be restored within 2 hours of its removal (Figure 2B).
These results suggest that the combination of dual Src/ABL inhibitors with blinatumomab may abrogate the effects of blinatumomab by directly inhibiting T-cell function. This is likely via inhibition of LCK, a known member of the TCR signaling pathway. This attribute of dasatinib has been recognized by other groups and has been suggested as a way to inhibit T-cell activation and may be useful as an on-off switch for chimeric antigen receptor T-cell therapy.9,10,14,20,21
Although small case series have reported responses in patients treated with blinatumomab and the dual Src/ABL inhibitors, it is possible that much of the response is from the TKI rather than blinatumomab. Preliminary results from the D-Alba trial presented at the 2019 Annual Meeting of the American Society of Hematology showed that, after 85 days of treatment with dasatinib and steroids (29.3% molecular response), the addition of blinatumomab led to a molecular response of 56.3% after 2 cycles.22 In contrast, a phase 2 study investigating the effects of blinatumomab treatment in patients with Ph− ALL who were minimal residual disease+ demonstrated that 78% achieved minimal residual disease negativity after 1 cycle of blinatumomab,23 and in a separate phase 2 study of patients with relapsed/refractory Ph+ ALL treated with blinatumomab monotherapy without addition of a TKI, 64% of those with <50% blasts achieved minimal residual disease negativity after 1 cycle of blinatumomab.6 Although comparisons across trials are fraught with challenges, it is possible that the addition of dasatinib to blinatumomab may not provide any added activity. Whether the short half-life of the dual Src/ABL TKIs will lead to restoration of blinatumomab’s efficacy during times of low plasma levels or whether the repeated interruptions of T-cell signaling and the lack of positive feedback promoted by cytokine production may lead to a blunted response to blinatumomab is unknown. Additionally, dasatinib can exert changes on the composition of immune cells over months, including promoting expansion of natural killer cells and cytotoxic T cells and reducing the number of T-regulatory cells,24,25 adding to complexities that are difficult to capture in the laboratory setting. Only a randomized trial of a TKI+/− blinatumomab would be able to discern whether there is a benefit to adding a dual Src/ABL TKI to bispecific antibody therapy vs using these agents in sequence. Although our data are limited by sample numbers and by the fact that responses in living subjects may differ according to many other complex interactions in the in vivo immune microenvironment, the potential immunomodulatory effects of targeted therapies should be taken into consideration when combined with immunotherapies.
For original data, please e-mail the corresponding author at leonard@ohsu.edu.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgment
Blinatumomab for in vitro use was kindly supplied by Amgen.
Authorship
Contribution: J.T.L., B.H.C., and E.L. designed the research; J.T.L., B.H.C., and E.L. wrote the paper; J.W.T., B.J.D., A.L., and B.H.-L. edited the paper; and Y.K., P.M., A.L., K.B., D.L., and J.T.L. performed the experiments.
Conflict-of-interest disclosure: J.T.L. received research funding from Amgen and was on the scientific advisory board for Takeda. B.J.D. was on the scientific advisory board for Aileron Therapeutics, Therapy Architects (ALLCRON), Cepheid, Vivid Biosciences, Celgene, RUNX1 Research Program, EnLiven Therapeutics, Gilead Sciences (inactive), and Monojul (inactive); was on the advisory board for and owns stock in Aptose Biosciences, Blueprint Medicines, Iterion Therapeutics, Third Coast Therapeutics, and GRAIL (SAB inactive); was the scientific founder of MolecularMD (inactive, acquired by ICON); was on the board of directors for and owns stock in Amgen; is on the board of directors for Burroughs Wellcome Fund and CureOne; is on the Joint Steering Committee for Beat AML LLS; is the founder of VB Therapeutics; receives funding from Novartis, Bristol-Myers Squibb, and Pfizer; and receives royalties from patent 6958335 (Novartis exclusive license), Oregon Health & Science University, and Dana-Farber Cancer Institute (1 Merck exclusive license). J.W.T. receives research support from Agios, Aptose, Array, AstraZeneca, Constellation, Genentech, Gilead, Incyte, Janssen, Petra, Seattle Genetics, Syros, and Takeda. E.L. receives research funding from Amgen, Celgene, Janssen Pharmaceuticals, Monojul (inactive), and Kyn Therapeutics. The remaining authors declare no competing financial interests.
Correspondence: Jessica Leonard, 3181 SW Sam Jackson Park Rd, Mail Code KR-HEM, Portland, OR 97239; e-mail: leonard@ohsu.edu.