In this issue of Blood, Leonard et al report that the dual Src/ABL inhibitors dasatinib and ponatinib inhibit in vitro blinatumomab-induced T-cell activation in blood samples from patients with Philadelphia chromosome positive acute lymphoblastic leukemia (Ph+ ALL).1
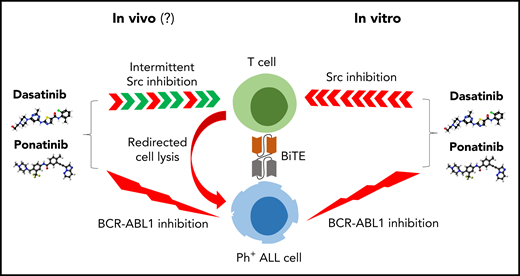
Right, dual ABL/Src inhibitors such as dasatinib and ponatinib inhibit blinatumomab-induced T-cell activation in vitro. Left, the in vivo effects on T cells may be less pronounced because of intermittent inhibition of Src kinases and additional effects of regulatory T cells. BiTE, bispecific T-cell engager.
Right, dual ABL/Src inhibitors such as dasatinib and ponatinib inhibit blinatumomab-induced T-cell activation in vitro. Left, the in vivo effects on T cells may be less pronounced because of intermittent inhibition of Src kinases and additional effects of regulatory T cells. BiTE, bispecific T-cell engager.
Patients with Ph+ ALL are typically treated with the combination of conventional chemotherapy and a tyrosine kinase inhibitor (BCR-ABL1 inhibitors). This approach is supported by a significant improvement in survival compared with chemotherapy alone.2 All available tyrosine kinase inhibitors (TKIs) have been evaluated from imatinib to second- and third-generation TKIs. No improvement in survival has been formally demonstrated favoring 1 TKI over the others. However, second- and third-generation TKIs induce a deeper reduction in minimal residual disease (MRD) levels after induction or during consolidation and ponatinib remains the only option if the T315I BCR-ABL1 kinase domain mutation is detected. The choice of the chemotherapeutic regimen will depend on age and fitness and ranges from full dose to reduced intensity regimens.3,4
Blinatumomab is a bispecific T-cell engager approved for relapsed or refractory ALL, including Ph+ ALL, and for treatment of MRD positivity after chemotherapy. The combination of blinatumomab and TKIs is a reasonable next step to pave the way toward chemo-sparing therapy in Ph+ ALL for induction or salvage therapy. Our enthusiasm for this approach may be tempered by the results presented here by Leonard et al. Using primary B cells from Ph+ ALL patients, they investigated whether dasatinib or ponatinib would prevent T-cell activation mediated by blinatumomab by altering Lck-dependent T-cell receptor signaling. As predicted, low concentrations (in the nM range) of dasatinib or ponatinib added to the culture with blinatumomab abrogated T-cell proliferation and interferon-γ production. This effect was not observed when TKIs without Src inhibitory potential such as imatinib or nilotinib were used.
One ongoing study from the GIMEMA reported the sequential use of dasatinib and blinatumomab as first-line induction and consolidation therapy in Ph+ ALL patients. The complete molecular response rate was up to 60%.5 A few studies reported on the clinical efficacy of TKIs given in combination with blinatumomab. Assi et al reported a retrospective analysis of 12 patients (9 patients with Ph+ ALL including 5 overt relapses and 4 positive MRD plus 3 chronic myeloid leukemia blast crisis patients including 1 overt relapse and 2 positive MRD) treated with blinatumomab and ponatinib (n = 8), dasatinib (n = 3), or bosutinib (n = 1). Among the 6 patients treated for overt hematologic disease, 4 (67%) responded (3 complete response and 1 partial response). Of the 6 patients who were treated for positive MRD, 6 attained negative MRD status.6 Similar results were reported by Hanif et al on 5 patients (2 overt relapses and 3 positive MRD) who received dasatinib or ponatinib with blinatumomab. All patients responded, including 3 complete molecular responses.7 A third retrospective study involved 11 patients with positive MRD treated with the combination of blinatumomab and ponatinib (n = 5), dasatinib (n = 4), imatinib (n = 1), or nilotinib (n = 1) and again complete molecular response was achieved in all evaluable patients.8 Finally, 15 patients with relapsed Ph+ ALL, including 4 with T315I mutation, received the combination of blinatumomab with continuous ponatinib. Complete remission was achieved in 14 patients with a complete molecular response in 12 patients.9
How can we reconcile the data from Leonard et al with these clinical observations? First, it is tempting to argue that most of the responses are from the TKIs rather than blinatumomab. However, the observed high level of responses is not in line with the disappointing efficacy of dasatinib or ponatinib monotherapy. Kinetics and duration of responses are more consistent with the known results of blinatumomab therapy. Second, the effects of dasatinib on T cells may be more complex with activity not only against cytotoxic T cell but also against regulatory T cells and CD4+ T-cell subsets.10 Third, the pharmacokinetic properties of dasatinib and ponatinib leading to transient off-target inhibition in vivo may lower the consequences of T-cell inhibition as also reported by Leonard et al when only short incubations of dasatinib were used (see figure).
First- and second-line trials are ongoing testing TKIs and blinatumomab given concomitantly (NCT02143414, NCT03147612, NCT03263572, NCT04530565) or sequentially (NCT02744768). The findings by Leonard et al must serve as a warning for those trials. We have now to move from “naïve” proposals combining innovative therapies to more complexity by examining synergistic, antagonistic, and time-dependent effects.
Conflict-of-interest disclosure: The author declares no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal