Key Points
The addition of caplacizumab to standard of care treatment in acquired thrombotic thrombocytopenic purpura is not cost effective compared with standard of care alone.
In a Markov model over a 5-year time horizon, the ICER for addition of caplacizumab to standard of care, compared with standard of care alone, is $1 482 260.
Abstract
Acquired thrombotic thrombocytopenic purpura (TTP) is a life-threatening disease characterized by thrombotic microangiopathy leading to end-organ damage. The standard of care (SOC) treatment is therapeutic plasma exchange (TPE) alongside immunomodulation with steroids, with increasing use of rituximab ± other immunomodulatory agents. The addition of caplacizumab, a nanobody targeting von Willebrand factor, was shown to accelerate platelet count recovery and reduce TPE treatments and hospital length of stay in TTP patients treated in 2 major randomized clinical trials. The addition of caplacizumab to SOC also led to increased bleeding from transient reductions in von Willebrand factor and increased relapse rates. Using data from the 2 clinical trials of caplacizumab, we performed the first-ever cost-effectiveness analysis in TTP. Over a 5-year period, the projected incremental cost-effectiveness ratio (ICER) in our Markov model was $1 482 260, significantly above the accepted 2019 US willingness-to-pay threshold of $195 300. One-way sensitivity analyses showed the utility of the well state and the cost of caplacizumab to have the largest effects on ICER, with a reduction in caplacizumab cost demonstrating the single greatest impact on lowering the ICER. In a probabilistic sensitivity analysis, SOC was favored over caplacizumab in 100% of 10 000 iterations. Our data indicate that the addition of caplacizumab to SOC in treatment of acquired TTP is not cost effective because of the high cost of the medication and its failure to improve relapse rates. The potential impact of caplacizumab on health system cost using longer term follow-up data merits further study.
Introduction
Acquired thrombotic thrombocytopenic purpura (TTP) is a rare disease (RD) and hematologic emergency characterized by end-organ damage in the setting of a thrombotic microangiopathy. Acquired TTP is caused by the development of autoantibodies directed against the von Willebrand factor-cleaving metalloproteinase ADAMTS13, leading to accumulation of thrombogenic ultra-large von Willebrand factor multimers and microvascular occlusion. In the absence of treatment, acquired TTP is uniformly fatal, but therapeutic plasma exchange (TPE) yields modern survival rates on the order of 85% or more.1,2
The autoantibody-mediated properties of acquired TTP form the basis for the use of immune suppression as adjunctive therapy in this disease, with the murine anti-CD20 monoclonal antibody rituximab being the most successful agent to date to show efficacy in reducing relapse rates.3-9 Other immune suppressive therapies used in acquired TTP include corticosteroids and, less often, mycophenolate mofetil, bortezomib, cyclophosphamide, and cyclosporine.10 The most recent addition to the TTP therapeutic armamentarium is caplacizumab, a humanized single-variable domain nanobody targeting the A1 domain of von Willebrand factor. Currently the only US Food and Drug Administration-approved medication for TTP, caplacizumab was shown in 2 clinical trials (Study to Assess Efficacy and Safety of Anti-von Willebrand Factor Nanobody in Patients With Acquired Thrombotic Thrombocytopenic Purpura [aTTP] [TITAN] and Phase III Trial With Caplacizumab in Patients With Acquired Thrombotic Thrombocytopenic Purpura [HERCULES]) to yield more rapid platelet count responses in acquired TTP when added to standard-of-care (SOC) therapy compared with SOC alone, albeit at the expense of increased bleeding rates because of transient reductions in von Willebrand factor levels and a significant increase in relapses following caplacizumab discontinuation.11,12
The impact of RDs on the health care system and their associated costs is significant and disproportionate to their prevalence.13-15 Therapies that are sufficiently effective, even if initially expensive, can still be beneficial for both RD patients and the health care system if they sufficiently alter the known natural history of the disease or improve quality of life. Cost-effectiveness analyses (CEA) have been instrumental in analyzing the societal impact of a number of therapies for RD and, in many cases, garnering support for expanding patient access to orphan drugs.16-20
With this in mind, we recently demonstrated that the use of rituximab in the treatment of initial or relapsed episodes of acquired TTP leads to hospital cost savings because of sufficient disease-modifying properties of rituximab in reducing long-term TTP relapse rates.21 Given the efficacy of caplacizumab in the TITAN and HERCULES trials, we conducted a CEA of caplacizumab in acquired TTP, representing the first-ever CEA in TTP.
Methods
Overview of models
We built decision tree models to evaluate the cost effectiveness of SOC plus caplacizumab vs SOC in acquired TTP based on the results of each of the phase 2 TITAN trial at 12-month follow-up and the phase 3 HERCULES trial at 1-month follow-up. We also created a Markov model comparing cost effectiveness of SOC plus caplacizumab vs SOC in acquired TTP based on the TITAN trial (Figure 1); we selected the TITAN trial rather than HERCULES for building the Markov model because of the comparatively longer follow-up period in the TITAN trial. We constructed our models using TreeAge Pro Healthcare 2020 (TreeAge Software, Williamstown, MA).
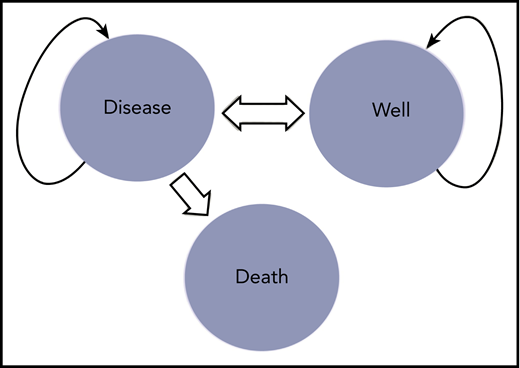
Markov model for TITAN trial. Shown are the various health states possible for patients enrolled on the TITAN trial, which include the disease state (ie, active TTP necessitating treatment), the well state (no active TTP), and death. All patients in the clinical trial start in the disease state, with some transitioning to the well state and some transitioning to death. Patients in the well state who have a recurrence will return back to the disease state, at which point the cycle starts again.
Markov model for TITAN trial. Shown are the various health states possible for patients enrolled on the TITAN trial, which include the disease state (ie, active TTP necessitating treatment), the well state (no active TTP), and death. All patients in the clinical trial start in the disease state, with some transitioning to the well state and some transitioning to death. Patients in the well state who have a recurrence will return back to the disease state, at which point the cycle starts again.
Assumptions
In all of our decision tree and Markov models, we assumed that the utility of either the well or the diseased state would be the same following treatment with SOC and caplacizumab vs SOC alone. We also assumed that TTP exacerbation (defined in the clinical trials as recurrence of TTP occurring within 30 days after completion of TPE) and relapse (defined as recurrence of thrombocytopenia arising after 30 days of completing daily TPE during a TTP episode) would be equally costly. This assumption serves to further favor the caplacizumab treatment arm, which demonstrated decreased exacerbations and increased relapses compared with SOC in both trials, as relapses are expected to be more costly than exacerbations because of the added risk of rehospitalization and a requirement for a higher level of care including central line replacement for TPE with the former. In the Markov model, transition-state cycles were 12 months in duration, with a total run of 5 cycles based on long-term follow-up data from studies of rituximab incorporated into SOC in acquired TTP.3,8,22
Base-case estimates and ranges for clinical probabilities used in our models are listed in Table 1. In all models, clinical probability of relapse or death was obtained from the TITAN and HERCULES trials data, whereas in the Markov model, for patients in the SOC group, we used a 5-year relapse probability range from 19% to 40% as derived from multiple published studies of rituximab incorporated into SOC in acquired TTP.3,8,22 To ensure that our analyses were unbiased toward the SOC arm, we used the absolute minimum total recurrence rates for caplacizumab for both trials and the absolute maximum values for total recurrence rates for SOC. Medical costs for both treatment arms in both trials were calculated from the health system perspective; future costs and effectiveness in the Markov model were discounted at a standard annual rate of 3%, a principle universal to cost-effectiveness models and based on the concept that the value of money and health in the present is more than their value in the future.23 Effectiveness refers to the cumulative total of utilities for each health state within each arm of each trial and was measured using quality-adjusted life years (QALYs) with the latter a product of the utility value of the state at 1 year. The primary outputs of these models were used to calculate the incremental cost-effectiveness ratio (ICER) in each model as the difference in total costs divided by the difference in effectiveness between the 2 treatment groups. Although a commonly accepted US willingness to pay (WTP) threshold of $150 000 per QALY gained has been used elsewhere, we opted to use the World Health Organization’s recommendation that WTP be 3 times the country’s gross domestic product (GDP), defined as the monetary value of all services and goods produced within a country’s borders over a specific period.23-25 Based on data from the International Monetary Fund DataMapper, the 2019 US GDP per capita is $65 110. For our study, we used 3 times the US GDP, amounting to a WTP of $195 330.
Base-case estimates and ranges
| Result or transition . | Estimate . | Range . | References . |
|---|---|---|---|
| TITAN | |||
| Cohort age at start, mean, y | 42 | 19-72 | 11 |
| Probability of death, SOC arm | 0.054 | 11 | |
| Probability of death, caplacizumab arm | 0 | 11 | |
| Probability of total recurrence SOC arm | 0.324 | 11 | |
| Probability of total recurrence, caplacizumab arm | 0.314 | 11 | |
| Probability of total relapse at 5 y | 0.4 | 0.19-0.4 | 3, 8, 22 |
| Discount rate | 0.03 | 0.015-0.06 | 23 |
| HERCULES | |||
| Cohort age at start, mean, y | 46 | 18-79 | 12 |
| Probability of death, SOC arm | 0.041 | 12 | |
| Probability of death caplacizumab arm | 0.014 | 12 | |
| Probability of total recurrence, SOC arm | 0.384 | 12 | |
| Probability of total recurrence, caplacizumab arm | 0.125 | 12 | |
| Discount rate | 0.03 | 0.015-0.06 | 23 |
| Result or transition . | Estimate . | Range . | References . |
|---|---|---|---|
| TITAN | |||
| Cohort age at start, mean, y | 42 | 19-72 | 11 |
| Probability of death, SOC arm | 0.054 | 11 | |
| Probability of death, caplacizumab arm | 0 | 11 | |
| Probability of total recurrence SOC arm | 0.324 | 11 | |
| Probability of total recurrence, caplacizumab arm | 0.314 | 11 | |
| Probability of total relapse at 5 y | 0.4 | 0.19-0.4 | 3, 8, 22 |
| Discount rate | 0.03 | 0.015-0.06 | 23 |
| HERCULES | |||
| Cohort age at start, mean, y | 46 | 18-79 | 12 |
| Probability of death, SOC arm | 0.041 | 12 | |
| Probability of death caplacizumab arm | 0.014 | 12 | |
| Probability of total recurrence, SOC arm | 0.384 | 12 | |
| Probability of total recurrence, caplacizumab arm | 0.125 | 12 | |
| Discount rate | 0.03 | 0.015-0.06 | 23 |
Utilities
Utilities were scaled from 0 to 1, with 0 set for the death state and 1 set for perfect health. We initially attempted to model utilities using existing resources including the comprehensive Cost Effectiveness Analysis Registry at Tufts (http://healtheconomicsdev.tuftsmedicalcenter.org/cear2/search/search.aspx), but there were no comparable reference diseases. Therefore, for our analyses patients who underwent successful treatment with resolution of TTP regardless of treatment modality were assumed to have a utility of 1, whereas those suffering relapse at any point were assigned a utility of 0.1. These utilities were selected because they provided the lowest ICER values, thereby minimizing bias against the caplacizumab arm and maximizing its cost effectiveness.
Costs
We assessed costs from the health system perspective. For cost of caplacizumab, we used the US list price ($270 000 for treatment of a typical episode of TTP). For cost of SOC, as previously published, we used the US average wholesale price for rituximab accounting for 4 inpatient doses ($7724 for each dose of 375 mg/m2 for a 170-cm, 70-kg individual) and our institution’s costs for number of TPE sessions ($6000 per TPE session), intensive care unit (ICU) length of stay (LOS) ($1043 per day for an ICU bed), and total hospital LOS ($490 per day for an inpatient general medicine bed).21 Because both clinical trials reported numerical ranges for number of TPE sessions and LOS, in our models we used the extremes of these ranges in order to maximize such costs in the SOC group and minimize costs in the caplacizumab group (supplemental Table, available on the Blood Web site). Because only HERCULES reported details on hospital LOS and ICU stay, we used these parameters for TITAN. We did not include costs associated with bleeding, which were seen at higher rates in the caplacizumab arms of both trials.
Sensitivity analyses
For each decision tree model, we performed 1-way sensitivity analyses varying the various model parameters individually to gauge their effects on the ICER. For the Markov model, in addition to a series of 1-way sensitivity analyses, we also performed a probabilistic sensitivity analysis (PSA), in which we varied all model parameters in numerous permutations and combinations, to assess the robustness of the model. For the probabilistic sensitivity analysis, we created distributions for clinical probabilities and health utilities using β-PERT distributions and used ɣ distributions for costs. Subsequently, we performed 10 000 Monte Carlo simulations, each randomly sampling from the entire distribution of model inputs, with clinical probabilities and health utilities represented by β distributions and costs represented by γ distributions.
Results
Decision tree analyses of TITAN and HERCULES trials
To analyze the cost effectiveness of adding caplacizumab to SOC in acquired TTP, we first performed decision tree analyses using data from the TITAN and HERCULES trials, which incorporated the total costs of caplacizumab and rituximab, total days of TPE, number of ICU days, total hospital LOS, TTP recurrence rates, and deaths in the caplacizumab and SOC treatment arms of both trials. The addition of caplacizumab to SOC yielded a higher cost of treatment compared with SOC alone in both trials (TITAN: $325 647 for caplacizumab plus SOC vs $89 750 for SOC; HERCULES: $323 547 for caplacizumab plus SOC vs $83 634 for SOC). An improvement in effectiveness with the addition of caplacizumab, as measured by QALYs, was noted as compared with SOC in both trials (TITAN: 0.72 for caplacizumab plus SOC vs 0.65 for SOC; HERCULES: 0.87 for caplacizumab plus SOC vs 0.61 for SOC). The ICER for adding caplacizumab to SOC vs SOC alone was $3 739 126 in the TITAN trial and $923 053 in the HERCULES trial.
Markov analysis of TITAN trial
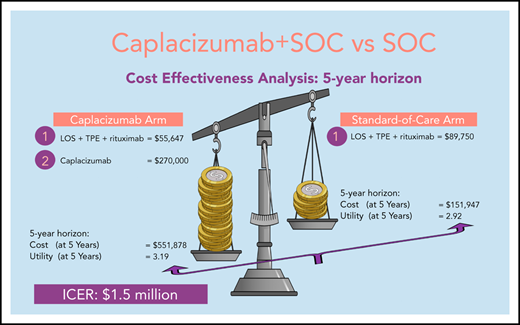
In a Markov model based on the TITAN trial, over a 5-year time horizon, the addition of caplacizumab to SOC yielded a higher cost of treatment compared with SOC alone at the conclusion of the model simulation ($551 878 for caplacizumab vs $151 947 for SOC). An improvement in QALYs with the addition of caplacizumab was noted as compared with SOC (3.19 for caplacizumab vs 2.92 for SOC). In this Markov analysis, the ICER for adding caplacizumab to SOC was $1 482 260.
Sensitivity analyses
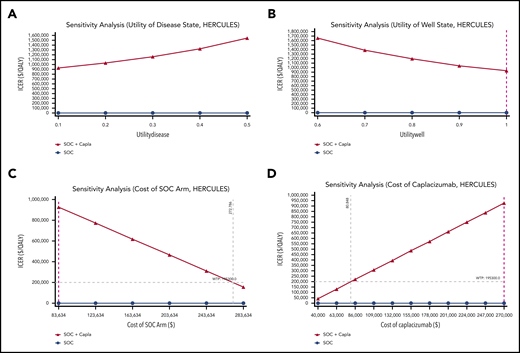
To identify which factors had the greatest impact on the cost effectiveness of adding caplacizumab to SOC, we performed 1-way sensitivity analyses using all parameters in our models for both the TITAN (Figure 2) and HERCULES (Figure 3) trials. Among the parameters of utility of the well and disease states, and costs of all components of the SOC arm and of caplacizumab itself, decreasing the cost of caplacizumab had the greatest impact on decreasing the ICERs of adding caplacizumab to SOC in both the TITAN and HERCULES trials. The price of caplacizumab treatment of 1 TTP episode to meet the 2019 US WTP would have to be $46 424 and $80 848 in the TITAN and HERCULES decision tree models, respectively. In this sensitivity analysis, at the current price of caplacizumab, the price of the SOC arm in both trials would have to more than triple to meet the WTP. Notably, improving the utility of the diseased state from 0.1 to 0.5 and decreasing the utility of the well state from 1.0 to 0.6 steadily increased the ICER in both trials.
One-way sensitivity analyses of TITAN trial with model parameters varied across ranges as shown. (A) Utility of well state. (B) Utility of disease state. (C) Cost of SOC arm. (D) Cost of caplacizumab. Purple dashed line represents baseline estimate.
One-way sensitivity analyses of TITAN trial with model parameters varied across ranges as shown. (A) Utility of well state. (B) Utility of disease state. (C) Cost of SOC arm. (D) Cost of caplacizumab. Purple dashed line represents baseline estimate.
One-way sensitivity analyses of HERCULES trial with model parameters varied across ranges as shown. (A) Utility of well state. (B) Utility of disease state. (C) Cost of SOC arm. (D) Cost of caplacizumab. Purple dashed line represents baseline estimate.
One-way sensitivity analyses of HERCULES trial with model parameters varied across ranges as shown. (A) Utility of well state. (B) Utility of disease state. (C) Cost of SOC arm. (D) Cost of caplacizumab. Purple dashed line represents baseline estimate.
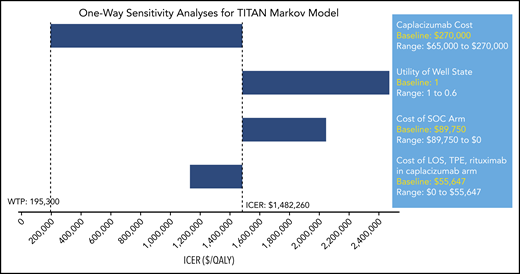
In a set of 1-way sensitivity analyses of the Markov model for the TITAN trial, the cost of caplacizumab itself again exerted a greater impact on the ICER of adding caplacizumab to SOC than any other factor analyzed, including number and cost of rituximab treatments, number of TPE days, and ICU and hospital LOS (Figure 4). According to this model, to meet the WTP, the cost of caplacizumab treatment of 1 episode of TTP would need to be $65 106, compared with its current list price of $270 000.
One-way sensitivity analyses for TITAN Markov. Each parameter is varied across a range shown with WTP and ICER delineated. Only parameters changing the ICER more or less than 10% are shown.
One-way sensitivity analyses for TITAN Markov. Each parameter is varied across a range shown with WTP and ICER delineated. Only parameters changing the ICER more or less than 10% are shown.
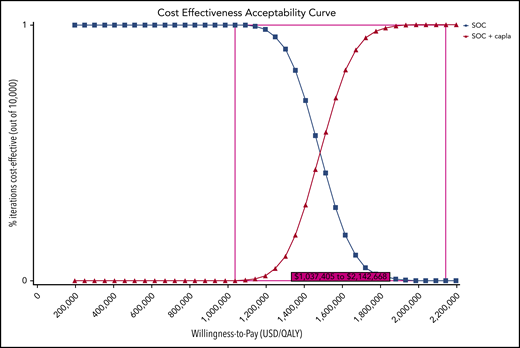
In a PSA of our Markov model for the TITAN trial, SOC was favored over SOC plus caplacizumab at a WTP of $195 330 in 100% of 10 000 iterations in a Monte Carlo simulation. In a cost-effectiveness acceptability curve, the addition of caplacizumab to SOC became the preferred strategy over SOC in 0.03% of 10 000 iterations starting at a minimum WTP of $1 037 405 and in 100% of 10 000 iterations at a minimum WTP of $2 142 668 (Figure 5). Based on PSA, we estimated an ICER of $1.46 million (95% confidence interval: $1.25-$1.72 million) (Table 2).
Cost-effectiveness acceptability curve. At a 2019 US WTP of $195 300, SOC is favored over SOC plus caplacizumab in 100% of 10 000 iterations. The ICER range at which caplacizumab is favored in 0.03% to 100% of iterations is USD $1 037 405 to $2 142 668. capla, caplacizumab.
Cost-effectiveness acceptability curve. At a 2019 US WTP of $195 300, SOC is favored over SOC plus caplacizumab in 100% of 10 000 iterations. The ICER range at which caplacizumab is favored in 0.03% to 100% of iterations is USD $1 037 405 to $2 142 668. capla, caplacizumab.
Baseline cost-effectiveness analysis and probabilistic sensitivity analysis
| Strategy . | Costs (US$) . | Incremental osts (US$) . | Effectiveness (QALY) . | Incremental effectiveness (QALY) . | ICER ($/QALY) . | ICER 95% CI ($/QALY) . |
|---|---|---|---|---|---|---|
| SOC | 155 806 | – | 3.16 | – | – | – |
| SOC + caplacizumab | 566 202 | 410 397 | 3.44 | 0.28 | 1 457 042 | 1 247 932-1 721 616 |
| Strategy . | Costs (US$) . | Incremental osts (US$) . | Effectiveness (QALY) . | Incremental effectiveness (QALY) . | ICER ($/QALY) . | ICER 95% CI ($/QALY) . |
|---|---|---|---|---|---|---|
| SOC | 155 806 | – | 3.16 | – | – | – |
| SOC + caplacizumab | 566 202 | 410 397 | 3.44 | 0.28 | 1 457 042 | 1 247 932-1 721 616 |
CI, confidence interval; costs, total costs of the standard of care and SOC + caplacizumab arms; effectiveness, added benefit in QALY of SOC and SOC + caplacizumab, using the assumptions described in the manuscript for utilities of the well and disease states; incremental costs, added cost of SOC + caplacizumab compared with SOC; incremental effectiveness, difference in effectiveness between SOC vs SOC + caplacizumab. The ICER is calculated by dividing incremental cost by incremental effectiveness.
Discussion
In this study, using data obtained from the TITAN and HERCULES trials including medication costs, TPE days, ICU and total hospital LOS, TTP recurrence rates, and deaths, we demonstrate that the addition of caplacizumab to SOC treatment in acquired TTP yields ICERs of ~$1 million to $4 million based on data obtained from the TITAN and HERCULES trials. This indicates that the addition of caplacizumab at its current list price is not cost effective compared with SOC in treating acquired TTP at a WTP of $195 330.
In our study, we incorporated a number of factors designed to maximize the cost effectiveness of caplacizumab and avoid potential confounders that might inadvertently favor SOC. The TITAN and HERCULES trials reported numerical ranges for number of TPE sessions and inpatient LOS; in our models, we chose the extremes of these ranges to maximize and minimize such costs in the SOC and caplacizumab groups, respectively. We did not include costs of bleeding, which occurred at increased frequency among caplacizumab-treated patients compared with SOC. In the TITAN trial, rituximab use was higher in the SOC arm than in caplacizumab-treated patients. Because utilities have not previously been established in acquired TTP patients, we calculated QALYs using hypothetical utility weights that augmented the difference between the well and diseased states to minimize the ICER and maximize the cost benefit of caplacizumab. Although in the 2 trials caplacizumab treatment was associated with increased TTP relapses and fewer exacerbations compared with SOC, in our models, we used a total recurrence rate representing the sum of relapses and exacerbations despite the former being more likely to incur greater costs as a result of hospital readmissions. Moreover, we used the absolute minimum and maximum total relapse rates for the caplacizumab and SOC arms, respectively. In addition, the US list price of caplacizumab that we used in our models ($270 000 per TTP episode) underestimated the total cost of the drug when taking into account the cost of each dose of caplacizumab ($8000) and the total number of days of caplacizumab treatment reported in the 2 clinical trials.26 Considering all of these factors, the ICER for adding caplacizumab to SOC in treating acquired TTP is likely to be higher than the values calculated in our models.
In 1-way sensitivity analyses, the variable with the single greatest impact on decreasing the ICER for caplacizumab treatment was lowering caplacizumab cost. Variations in other parameters, such as changes in SOC arm costs, hospital or ICU LOS, TPE days, or rituximab use, have far less of an impact than caplacizumab cost on overall cost effectiveness of adding caplacizumab to SOC in both decision trees and in our Markov model, whereas a decrease in the utility of the well state significantly increases the ICER. In all of our models, to meet the US WTP, a reduction in caplacizumab cost to a range of $46 424 to $80 848 per TTP episode lowered the ICER for caplacizumab more than any other variable analyzed (Figures 2,3-4).
Specific circumstances in which the addition of caplacizumab could potentially have a lower ICER would be in patients with severe (including cardiac and neurologic involvement) or refractory disease who are unable to leave the hospital because of a requirement for continued daily TPE. In the latter instance, caplacizumab could be used as rescue therapy to offset some of the costs incurred from prolonged hospitalizations. However, for this strategy to be cost effective at the accepted US WTP and the current list price of caplacizumab, the added hospital LOS and added days of TPE would have to more than triple (Figures 2C and 3C).
The ICERs calculated in our models for caplacizumab treatment in acquired TTP are much higher than either the generally accepted WTP threshold of $195 330 per QALY gained in the United States or the accepted cost-effectiveness thresholds for evaluating orphan drugs in RD in the United Kingdom and Sweden.24,27-31 Our cost-effectiveness acceptability curve shows that addition of caplacizumab is favored over SOC in 0.03% of cases starting at a minimum WTP of $1 037 405 and in 100% of cases starting at a WTP > $2 142 668.
To be sure, because of the high price of many orphan drugs, in CEAs of different RDs, the calculated ICERs for orphan drugs vary widely. In CEA studies of severe hemophilia A, both emicizumab and gene therapy in long-term follow-up are less costly and consequently dominate traditional factor VIII replacement therapy despite the hefty prices of these medications (up to $15 000-$30 000 per week for emicizumab, and $850 000 for 1 gene therapy treatment).19,20 By contrast, ICERs of well over $1 000 000 have been reported with eculizumab in treatment of paroxysmal nocturnal hemoglobinuria and enzyme replacement therapy in Gaucher and Pompe disease.18,32,33
A shared feature of many of these therapies is their ability to alter the natural history of the RDs being treated and potentially lower long-term costs associated with disease morbidity. Caplacizumab, by contrast, acts primarily in the setting of acute TTP; hence, its potential impact on the long-term course of acquired TTP and resultant costs is uncertain. If it is shown that the increasingly recognized long-term cognitive effects of TTP can be improved with the use of caplacizumab during a TTP flare, then caplacizumab would harbor an important disease-modifying property that would also help to decrease the ICER compared with SOC over the course of decades. On the other hand, if caplacizumab is associated with increased harm with respect to long-term outcomes, our study would be underestimating the ICER compared with the current SOC. Additional analyses of upcoming 36-month follow-up data from the HERCULES trial as well as real-world experience with caplacizumab will be important in further understanding the longer term cost implications of this medication.
Limitations of this study include short follow-up time for the 2 clinical trials upon which our decision tree models are based, the assumption that TTP exacerbations and relapses are of equal monetary cost, lack of established utilities in TTP for the healthy and disease states, unequal distribution of rituximab treatment in the TITAN trial, and exclusion of bleeding costs in our models. All modeling assumptions made in our analyses were designed to favor the cost effectiveness of caplacizumab. Despite these advantages, caplacizumab failed to meet the threshold for cost effectiveness in our study. In addition, we used the list prices for caplacizumab and rituximab, whereas real-life drug prices may vary according to insurance coverage and other factors. Nevertheless, only a steep reduction in the cost of caplacizumab would sufficiently reduce the ICER to meet 2019 US WTP standards, as recommended by the World Health Organization. Finally, we did not include theoretical added costs that would be incurred with more frequent ADAMTS13 monitoring to help prevent possible underpheresis with caplacizumab treatment, which would further increase the ICER for adding caplacizumab to SOC treatment in acquired TTP.
In summary, we performed the first-ever CEA in acquired TTP and found that the addition of caplacizumab to SOC treatment is not cost effective at its current drug pricing. Because our models are designed to maximize the cost effectiveness of caplacizumab, it is very likely that the actual costs incurred by this medication will be much higher than what we report here. Compared with CEA studies of other orphan drugs that, unlike caplacizumab, alter long-term disease course, the costs incurred by caplacizumab treatment in acquired TTP are at the higher end of the spectrum. Additional studies using longer term follow-up data are warranted to assess the full impact of caplacizumab on the cost of treating TTP.
For original data, please e-mail the corresponding author.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by a gift donation from Jack Levin to the Benign Hematology Program at Yale, grants from the National Institutes of Health National Institute of Allergy and Infectious Diseases (5T32AI052074-13) (P.S.) and National Institutes of Health National Heart, Lung, and Blood Institute (1K08 HL136840) (P.K.B.), and by the Luick Family Fund of Massachusetts General Hospital.
Authorship
Contribution: G.G. and A.I.L. conceived and designed the study; and G.G., P.S., J.E.H., C.T., P.K.B., and A.I.L. analyzed the data and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Alfred Ian Lee, Division of Hematology, Yale University School of Medicine, 333 Cedar St, New Haven CT 06520; e-mail: alfred.lee@yale.edu.


Comments
Response to Goshua et al (2020) "Cost effectiveness of caplacizumab in acquired thrombotic thrombocytopenic purpura"
US guidance for cost-effectiveness assessments recommends a lifetime horizon to reflect all key differences in costs and quality-adjusted life-years (QALYs) between treatment options [2]. As aTTP patients are relatively young at presentation (mean age mid-40s), modeling a lifetime horizon is particularly important and more appropriate than the short 5-year time horizon used.
The article does not describe how QALYs were calculated, which would help to understand the robustness of their cost-effectiveness results.
The authors do not acknowledge nor account for the fact that patients receiving placebo in the HERCULES trial received caplacizumab open-label in the event of disease exacerbations [3]. This will have a substantial impact on mortality and exacerbation outcomes estimated.
There are discrepancies in the relapse rates; the 5-year relapse rate of 19% cited for caplacizumab does not align with a 1.7-fold caplacizumab cost increase in the 5-year model versus the 2 short-term models. This suggests that a 5-year relapse rate of 70% was incorrectly used. Furthermore, the authors only considered the costs of relapse and not the composite benefits of caplacizumab use on relapse; i.e., reduction in mortality, thromboembolic events, or exacerbations.
Finally, assuming a utility of 1 (full-health) for patients in remission does not reflect the clinical burden of aTTP patients. Patients may suffer different levels of morbidity (depression, cognitive impairment, stroke, myocardial infarction) post-acute aTTP episode [4]. While we await long-term outcomes data, it is likely that caplacizumab-mediated reductions in microthrombi will lead to fewer long-term complications and potentially improve life expectancy [5].
Altogether, this assessment underestimates QALYs and overestimates costs. We believe if the authors adhered to accepted methodological guidance and addressed these limitations, their conclusions would likely align with other economic evaluations of caplacizumab [5].
Acknowledgements
No funding was provided for the writing of this letter. Pollissard is an employee and stockholder of Sanofi. Sullivan is a consultant for Sanofi.
References:
1. Goshua G, et al. Blood. 2020 Dec 6:blood.2020006052.
2. US Institute for Clinical and Economic Review. 2020-2023 Value Assessment Framework. https://icer.org/wp-content/uploads/2020/10/ICER_2020_2023_VAF_102220.pdf.
3. Scully M, et al. N Engl J Med. 2019;380(4):335-346.
4. Burns D, et al. Value Health. 2018; 21(Supp.3):S469.
5. Hughes M, et al. Lancet Haematol. 2021 Jan;8(1):e14-e15.
Corresponding Author:
Laurence Pollissard
1 Avenue Pierre Brossolette
91380 Chilly-Mazarin - France
E-mail: Laurence.Pollissard@sanofi.com
CEA of Caplacizumab might be more complex
1. Lee D, Thornton P, Hirst A, Kutikova L, Deuson R, Brereton N. Cost effectiveness of romiplostim for the treatment of chronic immune thrombocytopenia in Ireland. Applied health economics and health policy. 2013;11(5):457-469.
2. Kikuchi K, Miyakawa Y, Ikeda S, Sato Y, Takebayashi T. Cost-effectiveness of adding rituximab to splenectomy and romiplostim for treating steroid-resistant idiopathic thrombocytopenic purpura in adults. BMC health services research. 2015;15:2. Published January 22, 2015.
3. Völker LA, Kaufeld J, Miesbach W, et al. Real-world data confirm the effectiveness of caplacizumab in acquired thrombotic thrombocytopenic purpura. Blood advances. 2020;4(13):3085-3092.
4. Dutt T, Shaw RJ, Stubbs MJ, et al. Real-World Evidence of Caplacizumab Use in the Management of Acute TTP. Blood. 2020. Published November 4, 2020.
5. Völker LA, Kaufeld J, Miesbach W, et al. ADAMTS13 and VWF activities guide individualized caplacizumab treatment in patients with aTTP. Blood advances. 2020;4(13):3093-3101.