Skip Nav Destination
Thrombotic thrombocytopenic purpura and defective apoptosis due to CASP8/10 mutations: the role of mycophenolate mofetil
Second primary malignancies in ruxolitinib-treated myelofibrosis: real-world evidence from 219 consecutive patients
Posttransplant cyclophosphamide vs cyclosporin A and methotrexate as GVHD prophylaxis in matched sibling transplantation
Analysis of the cost-effectiveness of treatment strategies for CML with incorporation of treatment discontinuation
Overexpression of WT1 and PRAME predicts poor outcomes of patients with myelodysplastic syndromes with thrombocytopenia
Increased Mac-2 binding protein glycan isomer in patients at risk for late nonrelapse mortality after HSCT
Issue Archive
November 12 2019
In this Issue
Table of Contents
POINT-COUNTERPOINT
EXCEPTIONAL CASE REPORTS
Thrombotic thrombocytopenic purpura and defective apoptosis due to CASP8/10 mutations: the role of mycophenolate mofetil
Clinical Trials & Observations
F. Fioredda,E. Cappelli,A. Mariani,A. Beccaria,E. Palmisani,A. Grossi,I. Ceccherini,R. Venè,C. Micalizzi,M. Calvillo,F. Pierri,I. Mancini,F. Peyvandi,F. Corsolini,C. Dufour,M. Miano
STIMULUS REPORTS
Second primary malignancies in ruxolitinib-treated myelofibrosis: real-world evidence from 219 consecutive patients
Clinical Trials & Observations
Margherita Maffioli,Toni Giorgino,Barbara Mora,Alessandra Iurlo,Elena Elli,Maria Chiara Finazzi,Marianna Caramella,Elisa Rumi,Maria Cristina Carraro,Nicola Polverelli,Mariella D’Adda,Simona Malato,Marianna Rossi,Alfredo Molteni,Alessandro Vismara,Cinzia Sissa,Francesco Spina,Michela Anghilieri,Daniele Cattaneo,Rossella Renso,Marta Bellini,Maria Luisa Pioltelli,Chiara Cavalloni,Daniela Barraco,Raffaella Accetta,Lorenza Bertù,Matteo Giovanni Della Porta,Francesco Passamonti
Cranberry A-type proanthocyanidins selectively target acute myeloid leukemia cells
Laura M. Bystrom,Daniel P. Bezerra,Hsiao-Ting Hsu,Hongliang Zong,Luis A. Lara-Martínez,Jeanne P. De Leon,Megan Emmanuel,David Méry,Sara Gardenghi,Duane Hassane,Catherine C. Neto,Susanna Cunningham-Rundles,Michael W. Becker,Stefano Rivella,Monica L. Guzman
REVIEW ARTICLES
SYSTEMATIC REVIEW
CLINICAL TRIALS AND OBSERVATIONS
Posttransplant cyclophosphamide vs cyclosporin A and methotrexate as GVHD prophylaxis in matched sibling transplantation
Clinical Trials & Observations
Mi Kwon,on behalf of Grupo Español de Trasplante Hematopoyético y Terapia Celular (GETH),Rebeca Bailén,on behalf of Grupo Español de Trasplante Hematopoyético y Terapia Celular (GETH),María Jesús Pascual-Cascón,on behalf of Grupo Español de Trasplante Hematopoyético y Terapia Celular (GETH),Ana Isabel Gallardo-Morillo,on behalf of Grupo Español de Trasplante Hematopoyético y Terapia Celular (GETH),Abel García Sola,on behalf of Grupo Español de Trasplante Hematopoyético y Terapia Celular (GETH),Pascual Balsalobre,on behalf of Grupo Español de Trasplante Hematopoyético y Terapia Celular (GETH),Laura Solán,on behalf of Grupo Español de Trasplante Hematopoyético y Terapia Celular (GETH),Nieves Dorado,on behalf of Grupo Español de Trasplante Hematopoyético y Terapia Celular (GETH),Cristina Muñoz,on behalf of Grupo Español de Trasplante Hematopoyético y Terapia Celular (GETH),David Serrano,on behalf of Grupo Español de Trasplante Hematopoyético y Terapia Celular (GETH),Carolina Martínez-Laperche,on behalf of Grupo Español de Trasplante Hematopoyético y Terapia Celular (GETH),Ismael Buño,on behalf of Grupo Español de Trasplante Hematopoyético y Terapia Celular (GETH),Javier Anguita,on behalf of Grupo Español de Trasplante Hematopoyético y Terapia Celular (GETH),José Luis Díez-Martin,on behalf of Grupo Español de Trasplante Hematopoyético y Terapia Celular (GETH)
GENE THERAPY
Etranacogene dezaparvovec (AMT-061 phase 2b): normal/near normal FIX activity and bleed cessation in hemophilia B
Annette Von Drygalski,Adam Giermasz,Giancarlo Castaman,Nigel S. Key,Susan Lattimore,Frank W. G. Leebeek,Wolfgang Miesbach,Michael Recht,Alison Long,Robert Gut,Eileen K. Sawyer,Steven W. Pipe
Genome editing of HBG1 and HBG2 to induce fetal hemoglobin
Jean-Yves Métais,Phillip A. Doerfler,Thiyagaraj Mayuranathan,Daniel E. Bauer,Stephanie C. Fowler,Matthew M. Hsieh,Varun Katta,Sagar Keriwala,Cicera R. Lazzarotto,Kevin Luk,Michael D. Neel,S. Scott Perry,Samuel T. Peters,Shaina N. Porter,Byoung Y. Ryu,Akshay Sharma,Devlin Shea,John F. Tisdale,Naoya Uchida,Scot A. Wolfe,Kaitly J. Woodard,Yuxuan Wu,Yu Yao,Jing Zeng,Shondra Pruett-Miller,Shengdar Q. Tsai,Mitchell J. Weiss
HEALTH SERVICES AND OUTCOMES
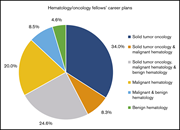
Associations between hematology/oncology fellows’ training and mentorship experiences and hematology-only career plans
Leah E. Masselink,Clese E. Erikson,Nathan T. Connell,Laura M. De Castro,Georgette A. Dent,Ariela L. Marshall,Rakhi P. Naik,Marquita Nelson,Casey L. O’Connell,Anita Rajasekhar,Deva Sharma,Melody Smith,Alfred Ian Lee
HEMATOPOIESIS AND STEM CELLS
Replication timing alterations in leukemia affect clinically relevant chromosome domains
Juan Carlos Rivera-Mulia,Takayo Sasaki,Claudia Trevilla-Garcia,Naoto Nakamichi,David J. H. F. Knapp,Colin A. Hammond,Bill H. Chang,Jeffrey W. Tyner,Meenakshi Devidas,Jared Zimmerman,Kyle N. Klein,Vivek Somasundaram,Brian J. Druker,Tanja A. Gruber,Amnon Koren,Connie J. Eaves,David M. Gilbert
IMMUNOBIOLOGY AND IMMUNOTHERAPY
Rational design of a trimeric APRIL-based CAR-binding domain enables efficient targeting of multiple myeloma
Andrea Schmidts,Maria Ormhøj,Bryan D. Choi,Allison O. Taylor,Amanda A. Bouffard,Irene Scarfò,Rebecca C. Larson,Matthew J. Frigault,Kathleen Gallagher,Ana P. Castano,Lauren S. Riley,Maria L. Cabral,Angela C. Boroughs,Rubí M.-H. Velasco Cárdenas,Wolfgang Schamel,Jing Zhou,Sean Mackay,Yu-Tzu Tai,Kenneth C. Anderson,Marcela V. Maus
LYMPHOID NEOPLASIA
Human MYD88L265P is insufficient by itself to drive neoplastic transformation in mature mouse B cells
Tomasz Sewastianik,Maria Luisa Guerrera,Keith Adler,Peter S. Dennis,Kyle Wright,Vignesh Shanmugam,Ying Huang,Helen Tanton,Meng Jiang,Amanda Kofides,Maria G. Demos,Audrey Dalgarno,Neil A. Patel,Anwesha Nag,Geraldine S. Pinkus,Guang Yang,Zachary R. Hunter,Petr Jarolim,Nikhil C. Munshi,Steven P. Treon,Ruben D. Carrasco
More precisely defining risk peri-HCT in pediatric ALL: pre- vs post-MRD measures, serial positivity, and risk modeling
Peter Bader,on behalf of the Children’s Oncology Group, the Pediatric Blood & Marrow Transplant Consortium, the Australian Transplantation Group, the International Berlin-Frankfurt-Münster Study Group, the Pediatric Diseases Working Party of the European Society for Blood and Marrow Transplantation, and the Westhafen Intercontinental Group,Emilia Salzmann-Manrique,on behalf of the Children’s Oncology Group, the Pediatric Blood & Marrow Transplant Consortium, the Australian Transplantation Group, the International Berlin-Frankfurt-Münster Study Group, the Pediatric Diseases Working Party of the European Society for Blood and Marrow Transplantation, and the Westhafen Intercontinental Group,Adriana Balduzzi,on behalf of the Children’s Oncology Group, the Pediatric Blood & Marrow Transplant Consortium, the Australian Transplantation Group, the International Berlin-Frankfurt-Münster Study Group, the Pediatric Diseases Working Party of the European Society for Blood and Marrow Transplantation, and the Westhafen Intercontinental Group,Jean-Hugues Dalle,on behalf of the Children’s Oncology Group, the Pediatric Blood & Marrow Transplant Consortium, the Australian Transplantation Group, the International Berlin-Frankfurt-Münster Study Group, the Pediatric Diseases Working Party of the European Society for Blood and Marrow Transplantation, and the Westhafen Intercontinental Group,Ann E. Woolfrey,on behalf of the Children’s Oncology Group, the Pediatric Blood & Marrow Transplant Consortium, the Australian Transplantation Group, the International Berlin-Frankfurt-Münster Study Group, the Pediatric Diseases Working Party of the European Society for Blood and Marrow Transplantation, and the Westhafen Intercontinental Group,Merav Bar,on behalf of the Children’s Oncology Group, the Pediatric Blood & Marrow Transplant Consortium, the Australian Transplantation Group, the International Berlin-Frankfurt-Münster Study Group, the Pediatric Diseases Working Party of the European Society for Blood and Marrow Transplantation, and the Westhafen Intercontinental Group,Michael R. Verneris,on behalf of the Children’s Oncology Group, the Pediatric Blood & Marrow Transplant Consortium, the Australian Transplantation Group, the International Berlin-Frankfurt-Münster Study Group, the Pediatric Diseases Working Party of the European Society for Blood and Marrow Transplantation, and the Westhafen Intercontinental Group,Michael J. Borowitz,on behalf of the Children’s Oncology Group, the Pediatric Blood & Marrow Transplant Consortium, the Australian Transplantation Group, the International Berlin-Frankfurt-Münster Study Group, the Pediatric Diseases Working Party of the European Society for Blood and Marrow Transplantation, and the Westhafen Intercontinental Group,Nirali N. Shah,on behalf of the Children’s Oncology Group, the Pediatric Blood & Marrow Transplant Consortium, the Australian Transplantation Group, the International Berlin-Frankfurt-Münster Study Group, the Pediatric Diseases Working Party of the European Society for Blood and Marrow Transplantation, and the Westhafen Intercontinental Group,Nathan Gossai,on behalf of the Children’s Oncology Group, the Pediatric Blood & Marrow Transplant Consortium, the Australian Transplantation Group, the International Berlin-Frankfurt-Münster Study Group, the Pediatric Diseases Working Party of the European Society for Blood and Marrow Transplantation, and the Westhafen Intercontinental Group,Peter J. Shaw,on behalf of the Children’s Oncology Group, the Pediatric Blood & Marrow Transplant Consortium, the Australian Transplantation Group, the International Berlin-Frankfurt-Münster Study Group, the Pediatric Diseases Working Party of the European Society for Blood and Marrow Transplantation, and the Westhafen Intercontinental Group,Allen R. Chen,on behalf of the Children’s Oncology Group, the Pediatric Blood & Marrow Transplant Consortium, the Australian Transplantation Group, the International Berlin-Frankfurt-Münster Study Group, the Pediatric Diseases Working Party of the European Society for Blood and Marrow Transplantation, and the Westhafen Intercontinental Group,Kirk R. Schultz,on behalf of the Children’s Oncology Group, the Pediatric Blood & Marrow Transplant Consortium, the Australian Transplantation Group, the International Berlin-Frankfurt-Münster Study Group, the Pediatric Diseases Working Party of the European Society for Blood and Marrow Transplantation, and the Westhafen Intercontinental Group,Hermann Kreyenberg,on behalf of the Children’s Oncology Group, the Pediatric Blood & Marrow Transplant Consortium, the Australian Transplantation Group, the International Berlin-Frankfurt-Münster Study Group, the Pediatric Diseases Working Party of the European Society for Blood and Marrow Transplantation, and the Westhafen Intercontinental Group,Lucia Di Maio,on behalf of the Children’s Oncology Group, the Pediatric Blood & Marrow Transplant Consortium, the Australian Transplantation Group, the International Berlin-Frankfurt-Münster Study Group, the Pediatric Diseases Working Party of the European Society for Blood and Marrow Transplantation, and the Westhafen Intercontinental Group,Gianni Cazzaniga,on behalf of the Children’s Oncology Group, the Pediatric Blood & Marrow Transplant Consortium, the Australian Transplantation Group, the International Berlin-Frankfurt-Münster Study Group, the Pediatric Diseases Working Party of the European Society for Blood and Marrow Transplantation, and the Westhafen Intercontinental Group,Cornelia Eckert,on behalf of the Children’s Oncology Group, the Pediatric Blood & Marrow Transplant Consortium, the Australian Transplantation Group, the International Berlin-Frankfurt-Münster Study Group, the Pediatric Diseases Working Party of the European Society for Blood and Marrow Transplantation, and the Westhafen Intercontinental Group,Vincent H. J. van der Velden,on behalf of the Children’s Oncology Group, the Pediatric Blood & Marrow Transplant Consortium, the Australian Transplantation Group, the International Berlin-Frankfurt-Münster Study Group, the Pediatric Diseases Working Party of the European Society for Blood and Marrow Transplantation, and the Westhafen Intercontinental Group,Rosemary Sutton,on behalf of the Children’s Oncology Group, the Pediatric Blood & Marrow Transplant Consortium, the Australian Transplantation Group, the International Berlin-Frankfurt-Münster Study Group, the Pediatric Diseases Working Party of the European Society for Blood and Marrow Transplantation, and the Westhafen Intercontinental Group,Arjan Lankester,on behalf of the Children’s Oncology Group, the Pediatric Blood & Marrow Transplant Consortium, the Australian Transplantation Group, the International Berlin-Frankfurt-Münster Study Group, the Pediatric Diseases Working Party of the European Society for Blood and Marrow Transplantation, and the Westhafen Intercontinental Group,Christina Peters,on behalf of the Children’s Oncology Group, the Pediatric Blood & Marrow Transplant Consortium, the Australian Transplantation Group, the International Berlin-Frankfurt-Münster Study Group, the Pediatric Diseases Working Party of the European Society for Blood and Marrow Transplantation, and the Westhafen Intercontinental Group,Thomas E. Klingebiel,on behalf of the Children’s Oncology Group, the Pediatric Blood & Marrow Transplant Consortium, the Australian Transplantation Group, the International Berlin-Frankfurt-Münster Study Group, the Pediatric Diseases Working Party of the European Society for Blood and Marrow Transplantation, and the Westhafen Intercontinental Group,Andre M. Willasch,on behalf of the Children’s Oncology Group, the Pediatric Blood & Marrow Transplant Consortium, the Australian Transplantation Group, the International Berlin-Frankfurt-Münster Study Group, the Pediatric Diseases Working Party of the European Society for Blood and Marrow Transplantation, and the Westhafen Intercontinental Group,Stephan A. Grupp,on behalf of the Children’s Oncology Group, the Pediatric Blood & Marrow Transplant Consortium, the Australian Transplantation Group, the International Berlin-Frankfurt-Münster Study Group, the Pediatric Diseases Working Party of the European Society for Blood and Marrow Transplantation, and the Westhafen Intercontinental Group,Michael A. Pulsipher,on behalf of the Children’s Oncology Group, the Pediatric Blood & Marrow Transplant Consortium, the Australian Transplantation Group, the International Berlin-Frankfurt-Münster Study Group, the Pediatric Diseases Working Party of the European Society for Blood and Marrow Transplantation, and the Westhafen Intercontinental Group
MYELOID NEOPLASIA
Analysis of the cost-effectiveness of treatment strategies for CML with incorporation of treatment discontinuation
Clinical Trials & Observations
Chihiro Yamamoto,Hirotomo Nakashima,Takashi Ikeda,Shin-ichiro Kawaguchi,Yumiko Toda,Shoko Ito,Kiyomi Mashima,Takashi Nagayama,Kento Umino,Daisuke Minakata,Hirofumi Nakano,Kaoru Morita,Ryoko Yamasaki,Miyuki Sugimoto,Yuko Ishihara,Masahiro Ashizawa,Kaoru Hatano,Kazuya Sato,Iekuni Oh,Shin-ichiro Fujiwara,Masuzu Ueda,Ken Ohmine,Kazuo Muroi,Yoshinobu Kanda
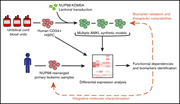
Human models of NUP98-KDM5A megakaryocytic leukemia in mice contribute to uncovering new biomarkers and therapeutic vulnerabilities
Sophie Cardin,Mélanie Bilodeau,Mathieu Roussy,Léo Aubert,Thomas Milan,Loubna Jouan,Alexandre Rouette,Louise Laramée,Patrick Gendron,Jean Duchaine,Hélène Decaluwe,Jean-François Spinella,Stéphanie Mourad,Françoise Couture,Daniel Sinnett,Élie Haddad,Josette-Renée Landry,Jing Ma,R. Keith Humphries,Philippe P. Roux,Josée Hébert,Tanja A. Gruber,Brian T. Wilhelm,Sonia Cellot
Overexpression of WT1 and PRAME predicts poor outcomes of patients with myelodysplastic syndromes with thrombocytopenia
Clinical Trials & Observations
Qiu-Sha Huang,Jing-Zhi Wang,Ya-Zhen Qin,Qiao-Zhu Zeng,Qian Jiang,Hao Jiang,Jin Lu,Hui-Xin Liu,Yi Liu,Jing-Bo Wang,Li Su,Hong-Yu Zhang,Zhen-Ling Li,Su-Jun Gao,Bo Huang,Yu-Ying Liu,Yan-Rong Liu,Lan-Ping Xu,Xiao-Jun Huang,Xiao-Hui Zhang
RED CELLS, IRON, AND ERYTHROPOIESIS
THROMBOSIS AND HEMOSTASIS
TRANSPLANTATION
Increased Mac-2 binding protein glycan isomer in patients at risk for late nonrelapse mortality after HSCT
Clinical Trials & Observations
Yu Akahoshi,Hideki Nakasone,Koji Kawamura,Machiko Kusuda,Shunto Kawamura,Junko Takeshita,Nozomu Yoshino,Yukiko Misaki,Kazuki Yoshimura,Ayumi Gomyo,Aki Tanihara,Masaharu Tamaki,Shun-ichi Kimura,Shinichi Kako,Yoshinobu Kanda
-
Cover Image
Cover Image
![issue cover]()
COVER FIGURE
Giemsa-stained touch preparation from the spleen showing infiltration of NUP98-KDM5A megakaryocytic leukemia cells in a secondary human xenograft recipient mouse 33 weeks after transplantation (original magnification ×1000) See the article by Cardin et al. - PDF Icon Front MatterFront Matter
- PDF Icon Editorial BoardEditorial Board
Advertisement intended for health care professionals
Advertisement intended for health care professionals