Key Points
We present real-world data on all ruxolitinib-treated myelofibrosis patients in a 10-million-resident region, with a follow-up of 2 years.
We found no evidence of an increased risk of developing lymphomas.
Introduction
Myeloproliferative neoplasms (MPNs) are clonal disorders that include polycythemia vera (PV), essential thrombocythemia (ET), and primary myelofibrosis (PMF). Patients with MPNs have a higher risk than the general population of developing a lymphoid neoplasm.1 It is still unclear whether this holds true for nonhematological second primary malignancies (SPMs).2-4 However, among 20 250 MPN patients included in the Surveillance, Epidemiology, and End Results (SEER) Program database, the 10-year cumulative incidence of SPMs was 12.7%, significantly higher than that expected in the general US population.5
Ruxolitinib (RUX) is an oral JAK inhibitor (JAKi) approved for International Prognostic Scoring System (IPSS)/Dynamic IPSS (DIPSS) intermediate- and high-risk myelofibrosis (MF)6,7 and for inadequately controlled PV. More than 2600 RUX-treated MF patients have been prospectively observed for at least 2 years within the 2 pivotal COMFORT trials8,9 and the expanded-access JUMP trial.10,11 Safety data from these trials underline a possibly increased incidence of nonmelanoma skin cancers (NMSCs), but no significant increase of lymphoproliferative neoplasms, similarly to what occurs in PV.12,13
Recently, Porpaczy et al alerted, however, on the possible 16-fold increased risk of developing aggressive lymphomas in MPN patients treated with JAKis, especially in the presence of a preexisting B-cell clone.14 The publication included a total of 1555 MPN patients, 126 of whom were treated with a JAKi (ruxolitinib, gandotinib, fedratinib, momelotinib), obtained assembling 2 broad academic data sets. In the well-described Viennese cohort, 3 of 31 MF patients treated with JAKi developed lymphomas. Median time from JAKi initiation to lymphoma diagnosis was 25 months.
Subsequent analyses of other large academic data sets did, however, not confirm an increased risk of aggressive lymphoma development under JAKis in MPNs15,16 and in post-PV and post-ET MF (secondary MF [SMF]).17 These contradictory results were derived either from clinical trials with strict eligibility criteria, possibly at the expense of uncertainty about the generalizability of results, or from highly selected data sets of patients evaluated at referral centers, thus highlighting the need for real-world data (RWD).
We consequently set out to assess the occurrence of SPMs, including lymphoproliferative neoplasms, in RUX-treated MF patients on the basis of RWD provided by the health authority of the Lombardy Region, integrated with institutional data.
Methods
The Italian health care service is regionally based and characterized by universal coverage. Drugs are delivered by each regional health authority, and most newly approved high-cost treatments, like RUX, are subject to longitudinal monitoring. This allows for an accurate identification of all individual patient prescriptions in the region. In addition, Lombardy, a region of roughly 10 million individuals, has a well-established network for hematology (Rete Ematologica Lombarda [REL]), with a specific commission for MPNs. Within the REL, and on the basis of RWD provided by the health authority of the Lombardy region, we conducted an ambispective observational study (RUXOREL-MF, clinicaltrials.gov identifier NCT03959371) including all MF patients treated with RUX provided by the regional health care system, that is, 219 subjects regularly followed at 16 centers. These data have been integrated with clinical and genetic data from each center.
The study was approved by the review board of each institution and conducted in accordance with the Declaration of Helsinki. Incidence rates were computed from the number of events vs the time at risk after RUX start. Comparisons with the general population were performed via Poisson models on age- and sex-matched incidence ratios, standardized with respect to available population data for Lombardy (2003-2007) provided in Cancer Incidence in Five Continents Volume X (CI5-X) tables (International Agency for Research on Cancer).18 Comparisons with RUX-naive MF patients (n = 246, derived from the Varese institutional database; main patient characteristics reported in supplemental Table 1) were performed via Poisson models with adjusted incidence rates. Time-to-event analyses were performed using Aalen-Johansen estimators of cumulative incidences, log-rank tests, and cause-specific Cox models based on time elapsed after RUX start.
Results and discussion
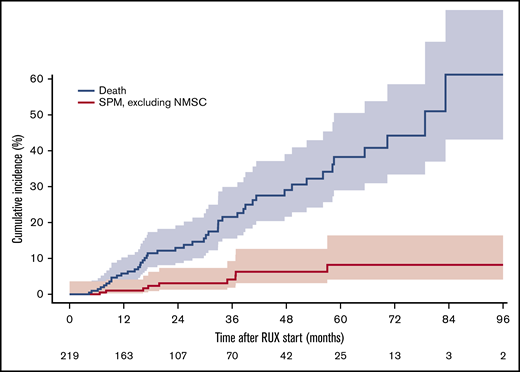
Demographics of the 219 patients treated with RUX and included in the study are summarized in Table 1. Among these, we recorded 23 SPMs (of which 13 NMSCs) in 18 subjects (5 patients experienced 2 SPMs). A direct comparison of main patient characteristics according to occurrence of an SPM is also summarized in Table 1. Median follow-up from diagnosis was 58.8 months (interquartile range [IQR], 33.9-96.4) and median duration of MF before RUX start was 30.1 months (IQR, 5.7-62.2). Median follow-up from RUX start was 24.0 months (IQR, 12.3-40.4), whereas median RUX exposure was 18.2 months (IQR, 8.3-37.2). At the time of data cutoff, that is, 4 June 2019, 146 patients (66.7%) are still on treatment and 50 died (23.0%). Cause of death was related to the SPM in 2 patients. Median estimated overall survival from diagnosis and from RUX start was 240.0 and 78.7 months, respectively. Distribution of SPMs during the course of the disease was as follows: 24 cases were reported prior to RUX start (no case of lymphoma) and 23 cases occurred after RUX start (lung, 1; breast, 2; prostate, 2; bladder, 1; melanoma, 1; multiple myeloma, 1; chronic myeloid leukemia, 1; hepatobiliary carcinoma, 1; NMSC, 13). None of the 219 RUX-treated patients developed a lymphoma. The incidence rate of SPMs post-RUX was 4.3 per 100 patient-years (95% confidence interval [CI], 2.7-6.4), whereas it was 1.9 per 100 patient-years (95% CI, 0.9-3.4), when excluding NMSCs from the analysis. Median time to SPM diagnosis after RUX start was 16.9 months (IQR, 6.9-21.5) and 19.8 months (IQR, 16.3-36.7) when excluding NMSCs. Cumulative incidence of SPMs (excluding NMSCs) after RUX start is reported in Figure 1. Patients with an SPM pre-RUX were at higher risk of developing an SPM post-RUX (hazard ratio [HR], 3.6; 95% CI, 1.2-11.2; P = .02). Significance was lost if the analysis excluded NMSCs (HR, 3.8; 95% CI, 0.8-19.0; P = .08). The incidence of SPMs in our cohort was significantly higher than the incidence of cancer in the general population (standardized incidence ratio: 1.7; 95% CI, 1.02-2.6; P = .026). The trend holds true for both males and female patients, although without statistical significance likely due to the smaller number of cases. Importantly, there is no significant difference if the analysis excludes NMSCs (namely ICD-10-CM diagnosis code C44; HR, 1.0; 95% CI, 0.5-1.8; P = 1.0). Finally, when comparing the incidence of SPMs in our RWD collection with that in 246 JAKi-naive MF patients, we found a trend toward an enrichment of SPMs in the RUX-treated group (4.3 vs 2.3 per 100 patient-years, rate ratio test: ratio, 1.84; 95% CI, 0.99-3.43; P = .06), which again ceased to be evident when NMSCs were excluded from the analysis (1.9 vs 1.3 per 100 patient-years; ratio, 1.41; 95% CI, 0.56-3.49; P = .53). Even after adjusting the comparison for type of diagnosis (PMF vs SMF) and for disease severity (IPSS for PMF and Myelofibrosis Secondary to PV and ET–Prognostic Model [MYSEC-PM] for SMF), we did not find a significant difference in SPM incidence between RUX-treated and RUX-naive patients (rate difference, 1.08 per 100 patient-years; P = .41; supplemental Results).
Main patient characteristics in the whole cohort and according to post-RUX SPM occurrence
| . | All, n = 219 . | No SPM post-RUX, n = 201 . | SPM post-RUX, excluding NMSC, n = 9 . | NMSC post-RUX, n = 9 . |
|---|---|---|---|---|
| Sex, M/F | 129/90 | 116/85 | 6/3 | 7/2 |
| Median age at diagnosis (range), y | 62 (19-83) | 61 (19-83) | 62 (46-69) | 67 (35-73) |
| Diagnosis | ||||
| PMF | 99 | 90 | 6 | 3 |
| Post-ET MF | 37 | 34 | 2 | 1 |
| Post-PV MF | 83 | 77 | 1 | 5 |
| Mutational status | ||||
| JAK2 mutated | 160 | 147 | 4 | 9 |
| CALR mutated | 33 | 30 | 3 | 0 |
| MPL mutated | 5 | 4 | 1 | 0 |
| Triple negative | 7 | 6 | 1 | 0 |
| Comutated | 3 | 3 | 0 | 0 |
| NA* | 11 | 11 | 0 | 0 |
| IPSS score at diagnosis (for PMF patients only, available in 89) | ||||
| Low | 27 | 26 | 1 | 0 |
| Intermediate-1 | 20 | 18 | 0 | 2 |
| Intermediate-2 | 22 | 19 | 2 | 1 |
| High | 20 | 19 | 1 | 0 |
| MYSEC-PM score at diagnosis (for SMF patients only, available in 107) | ||||
| Low | 26 | 25 | 1 | 0 |
| Intermediate-1 | 54 | 48 | 1 | 5 |
| Intermediate-2 | 20 | 18 | 1 | 1 |
| High | 7 | 7 | 0 | 0 |
| Median time from diagnosis to RUX start (range), mo | 30 (0-294) | 30 (0-294) | 30 (8-149) | 38 (1-59) |
| At RUX start | ||||
| Median age (range), y | 66 (24-84) | 65 (24-84) | 67 (49-74) | 70 (67-78) |
| Median WBC (range), ×109/L | 11.0 (1.8-70.0) | 11.1 (1.8-70.0) | 12.9 (5.3-31.1) | 10.3 (4.2-23.3) |
| Median Hb (range), g/dL | 10.4 (5.8-16.0) | 10.4 (5.8-16.0) | 10.0 (8.8-12.4) | 11.1 (8.9-13.5) |
| Median PLT (range), ×109/L | 213 (14-1425) | 212 (14-1425) | 387 (75-503) | 249 (61-570) |
| Median peripheral blood blasts (range), % | 1 (0-12) | 1 (0-12) | 2 (0-6) | 1 (0-6) |
| Constitutional symptoms, absent/present/NA | 72/141/6 | 67/129/5 | 2/7/0 | 3/5/1 |
| Median palpable splenomegaly from lcm (range), cm | 10.0 (0.0-34.0) | 10.0 (0.0-34.0) | 9.5 (5.0-24.0) | 10.0 (5.0-19.0) |
| DIPSS score, for PMF patients only, available in 95 | ||||
| Low | 3 | 3 | 0 | 0 |
| Intermediate-1 | 36 | 36 | 0 | 0 |
| Intermediate-2 | 41 | 34 | 5 | 2 |
| High | 15 | 13 | 1 | 1 |
| Median follow-up after RUX start (range), mo | 24 (0-110) | 23 (0-110) | 31 (12-75) | 39 (7-56) |
| Median RUX exposure (range), mo | 18 (0-110) | 17 (0-110) | 31 (12-75) | 39 (7-56) |
| SPM pre-RUX, all types | 20 | 16 | 3 | 1 |
| SPM pre-RUX, excluding NMSC | 16 | 14 | 2 | 0 |
| . | All, n = 219 . | No SPM post-RUX, n = 201 . | SPM post-RUX, excluding NMSC, n = 9 . | NMSC post-RUX, n = 9 . |
|---|---|---|---|---|
| Sex, M/F | 129/90 | 116/85 | 6/3 | 7/2 |
| Median age at diagnosis (range), y | 62 (19-83) | 61 (19-83) | 62 (46-69) | 67 (35-73) |
| Diagnosis | ||||
| PMF | 99 | 90 | 6 | 3 |
| Post-ET MF | 37 | 34 | 2 | 1 |
| Post-PV MF | 83 | 77 | 1 | 5 |
| Mutational status | ||||
| JAK2 mutated | 160 | 147 | 4 | 9 |
| CALR mutated | 33 | 30 | 3 | 0 |
| MPL mutated | 5 | 4 | 1 | 0 |
| Triple negative | 7 | 6 | 1 | 0 |
| Comutated | 3 | 3 | 0 | 0 |
| NA* | 11 | 11 | 0 | 0 |
| IPSS score at diagnosis (for PMF patients only, available in 89) | ||||
| Low | 27 | 26 | 1 | 0 |
| Intermediate-1 | 20 | 18 | 0 | 2 |
| Intermediate-2 | 22 | 19 | 2 | 1 |
| High | 20 | 19 | 1 | 0 |
| MYSEC-PM score at diagnosis (for SMF patients only, available in 107) | ||||
| Low | 26 | 25 | 1 | 0 |
| Intermediate-1 | 54 | 48 | 1 | 5 |
| Intermediate-2 | 20 | 18 | 1 | 1 |
| High | 7 | 7 | 0 | 0 |
| Median time from diagnosis to RUX start (range), mo | 30 (0-294) | 30 (0-294) | 30 (8-149) | 38 (1-59) |
| At RUX start | ||||
| Median age (range), y | 66 (24-84) | 65 (24-84) | 67 (49-74) | 70 (67-78) |
| Median WBC (range), ×109/L | 11.0 (1.8-70.0) | 11.1 (1.8-70.0) | 12.9 (5.3-31.1) | 10.3 (4.2-23.3) |
| Median Hb (range), g/dL | 10.4 (5.8-16.0) | 10.4 (5.8-16.0) | 10.0 (8.8-12.4) | 11.1 (8.9-13.5) |
| Median PLT (range), ×109/L | 213 (14-1425) | 212 (14-1425) | 387 (75-503) | 249 (61-570) |
| Median peripheral blood blasts (range), % | 1 (0-12) | 1 (0-12) | 2 (0-6) | 1 (0-6) |
| Constitutional symptoms, absent/present/NA | 72/141/6 | 67/129/5 | 2/7/0 | 3/5/1 |
| Median palpable splenomegaly from lcm (range), cm | 10.0 (0.0-34.0) | 10.0 (0.0-34.0) | 9.5 (5.0-24.0) | 10.0 (5.0-19.0) |
| DIPSS score, for PMF patients only, available in 95 | ||||
| Low | 3 | 3 | 0 | 0 |
| Intermediate-1 | 36 | 36 | 0 | 0 |
| Intermediate-2 | 41 | 34 | 5 | 2 |
| High | 15 | 13 | 1 | 1 |
| Median follow-up after RUX start (range), mo | 24 (0-110) | 23 (0-110) | 31 (12-75) | 39 (7-56) |
| Median RUX exposure (range), mo | 18 (0-110) | 17 (0-110) | 31 (12-75) | 39 (7-56) |
| SPM pre-RUX, all types | 20 | 16 | 3 | 1 |
| SPM pre-RUX, excluding NMSC | 16 | 14 | 2 | 0 |
DIPSS, Dynamic International Prognostic Scoring System; ET, essential thrombocythemia; F, female; Hb, hemoglobin; IPSS, International Prognostic Scoring System; lcm, left costal margin; M, male; MF, myelofibrosis; MYSEC-PM, Myelofibrosis Secondary to PV and ET–Prognostic Model; NA, not available; NMSC, nonmelanoma skin cancer; PLT, platelet; PMF, primary myelofibrosis; PV, polycythemia vera; RUX, ruxolitinib; SMF, secondary myelofibrosis; SPM, second primary malignancy; WBC, white blood cell.
NA includes patients who have tested 1 or 2 driver mutations and resulted unmutated but cannot be defined "triple negative" (TN) because not all 3 driver mutations have been studied, and patients for whom the driver mutational status is not available.
In conclusion, our RWD offer a unique opportunity to provide real-world evidence on the incidence of SPMs in MF patients receiving RUX. In our cohort, no case of lymphoma was registered with a median follow-up after RUX start, which, albeit relatively short, matches the median time to lymphoma occurrence reported by Porpaczy et al. Furthermore, when excluding NMSCs, RUX-treated MF patients do not seem to be at a higher risk of SPMs with respect to the general population and to a cohort of RUX-naive MF patients.
Acknowledgment
The Varese group was supported by the Fondazione Regionale Ricerca Biomedica, Milan, Italy (FRRB project no. 2015-0042, Genomic profiling of rare hematologic malignancies, development of personalized medicine strategies, and their implementation into the Rete Ematologica Lombarda [REL] clinical network), by the Fondazione Matarelli (Milan, Italy), the Fondazione Rusconi (Milan, Italy), and Associazione italiana contro le leucemie-linfomi e mieloma (AIL) Varese Organizzazione Non Lucrativa di Utilità Sociale (ONLUS).
Authorship
Contribution: M.M. and F.P. contributed to the conception and design of the work and wrote the paper; T.G. contributed to the design of the work and performed the statistical analysis; L.B. performed part of the statistical analysis; M.M., B.M., A.I., E.E., M.C.F., M.C., E.R., M.C.C., N.P., M.D., S.M., M.R., A.M., A.V., C.S., F.S., M.A., D.C., R.R., M.B., M.L.P., C.C., D.B., R.A., M.G.D.P., and F.P. contributed data collection; and T.G., B.M., A.I., E.E., M.C.F., M.C., E.R., M.C.C., N.P., M.D., S.M., M.R., A.M., A.V., C.S., F.S., M.A., D.C., R.R., M.B., M.L.P., C.C., D.B., R.A., L.B., and M.G.D.P. contributed to revising the manuscript critically for important intellectual content and approved the final version of the manuscript.
Conflict-of-interest disclosure: A.I. received speaker honoraria from Novartis, Pfizer, and Incyte. E.R. received consultancy fees from Novartis. F.P. received speaker honoraria from Novartis and Celgene, and participated in advisory boards for Celgene, Novartis, Roche, Janssen, Incyte, and AbbVie. The remaining authors declare no competing financial interests.
Correspondence: Francesco Passamonti, Department of Medicine and Surgery, University of Insubria, ASST Sette Laghi, Viale L. Borri 57, 21100 Varese, Italy; e-mail: francesco.passamonti@uninsubria.it.
References
Author notes
Individual participant data will not be shared.
The full-text version of this article contains a data supplement.