Key Points
PTCy with additional immunosuppression using mostly PBSCs grafts showed a reduction of acute GVHD rate in matched sibling donor HSCT.
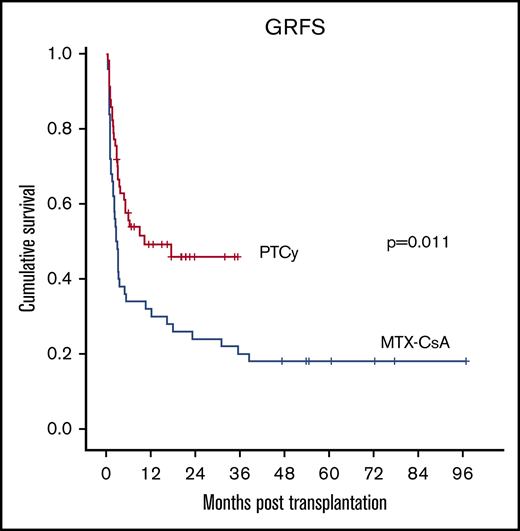
GRFS was improved after PTCy compared with CsA-MTX.
Abstract
Posttransplant cyclophosphamide (PTCy) effectively prevents graft-versus-host disease (GVHD) after HLA-haploidentical hematopoietic stem cell transplantation (HSCT). The use of PTCy in HLA-identical HSCT is less explored. We conducted a retrospective study of 107 consecutive patients undergoing an HLA-identical sibling (10/10) HSCT in 2 centers in Spain, 50 with GVHD prophylaxis with methotrexate–cyclosporin A (MTX-CsA) and 57 using a PTCy-based regimen with additional immunosuppression. Graft source was unmanipulated mobilized peripheral blood stem cells (PBSC) in most patients (97 patients, 91%). Cumulative incidences of grade II to IV and III to IV acute GVHD at 100 days were lower in the PTCy group (22.6% vs 52.2%, P = .0015; 8.8% vs 24.4%, P = .016), without statistically significant differences in the 2-year cumulative incidence of chronic moderate to severe GVHD (16.7% vs 26%, P = .306). At 2 years, no statistically significant differences were observed in OS (78% vs 56%, P = .088), EFS (62.5% vs 48%, P = .054), relapse (28% vs 27%, P = .47), and NRM (8.8% vs 24%, P = .054). The composite endpoint of GVHD and relapse-free survival (GRFS) was favorable for the PTCy group (24% vs 48%, P = .011), PTCy being the sole independent factor identified in the multivariate analysis for this endpoint. In this study, PTCy combination with additional immunosuppression using mostly PBSCs grafts showed a reduction of acute GVHD rate and an impact on GRFS, with safety results comparable with those obtained with MTX-CsA. Further prospective studies are needed to confirm these observations..
Introduction
Graft-versus-host disease (GVHD) is a major complication after allogeneic hematopoietic stem cell transplantation (HSCT) resulting in variable degrees of morbidity and quality-of-life compromise after transplantation in patients with long-term survival as well as higher rates of mortality.1,2 Since the introduction of the combination of methotrexate (MTX) and cyclosporin A (CsA) in the 1980s, the standard GVHD prophylaxis in HLA-matched sibling donor (MSD) transplantation is still based on the use of calcineurin inhibitors (CNIs) with either MTX or mycophenolate mofetil (MMF).3,4 This strategy results in 20% to 40% rates of significant acute (aGVHD) and chronic GVHD (cGVHD) contributing as the main cause of nonrelapse mortality (NRM) after HSCT from MSD. In the haploidentical transplantation setting, the use of high-dose posttransplant cyclophosphamide (PTCy) in combination with tacrolimus and MMF pioneered by Luznik et al results in low rates of aGVHD and cGVHD and consequently low rates of NRM.5 Furthermore, a number of studies have demonstrated the feasibility and efficacy of PTCy as a single agent for GVHD prophylaxis in HLA-matched HSCT with bone marrow (BM) as graft source.6,7 However, administration of single-agent PTCy in the setting of peripheral blood stem cell (PBSC) grafts from either HLA-identical sibling or matched unrelated donors resulted in unacceptable severe aGVHD rates and related deaths.8,9 However, the combination of PTCy with CsA was shown to decrease the incidence of severe forms of aGVHD after HLA-matched mobilized blood cell transplantation.10 Similarly, registry comparative studies suggested that the addition of immunosuppressive drugs to PTCy may enhance its effect, preventing severe cGVHD, reducing mortality, and improving survival after MSD and unrelated HSCT.11 Thus, different risk-adapted approaches based on PTCy plus additional immunosuppression have been described in this setting.12
The aim of this study was to analyze the results of the use of PTCy in combination with other immunosuppressive drugs, such as GVHD prophylaxis in MSD HSCT compared with CsA and MTX within the Spanish Group of Hematopoietic Stem Cell Transplantation (GETH).
Patients and methods
Patients
The PTCy group included 57 consecutive adult patients with hematological malignancies transplanted with a MSD in 2 centers from the Spanish Group of Hematopoietic Stem Cell Transplantation (GETH) between 2014 and 2017. The CsA-MTX group included 50 consecutive patients from one of the centers transplanted between 2010 and 2015. Due to the change in the prophylaxis strategy in both institutions for all MSD transplants, the comparative group was not contemporaneous. Patients included had a minimum posttransplantation follow-up of 6 months. The study was approved by each center’s ethical committee, and all patients signed informed consent.
GVHD prophylaxis in the PTCy group consisted of IV cyclophosphamide (Cy) 50 mg/kg at days +3 and +5 combined with CsA 5 mg/kg per day from day 0 (38 patients, 65%) or Cy 50 mg/kg at days +3 and +4 combined with CsA 5 mg/kg per day from day +5 and MMF 10 mg/kg every 8 hours from day +5 until day +35 (19 patients, 35%).5,13 CsA dose was decreased from day +60 and withdrawn by day +100 in the absence of GVHD.
GVHD prophylaxis in the historical control group included conventional CNI prophylaxis with CsA 5 mg/kg per day from day −1 and MTX 15 mg/m2 on day +1 and 10 mg/m2 on days +3, +6, and +11. Twenty-two patients (44%) received only the first 3 doses of MTX due to toxicity or very high risk of relapse as per center protocol. CsA was withdrawn by day +100 in the absence of GVHD.
The most common myeloablative conditioning (MAC) regimens in both groups included fludarabine (Flu) and IV busulfan (Bu) (Flu 30 mg/m2 per day days −6 to −3 and Bu 3.2 mg/kg per day days −6 to −3) or thiotepa (T), Bu, and Flu (TBF-MAC) (T 5 mg/kg per day days −7 to −6, Bu 3.2 mg/kg per day days −6 to −3, Flu 50 mg/m2 per day days −5 to −3). Reduced-intensity conditioning (RIC) was performed with Flu, Cy, and Bu (Flu 30 mg/m2 per day from days −6 to −2, Cy 14.5 mg/kg per day days −6 and −5, IV Bu 3.2 mg/kg per day days −3 and −2) or TBF-RIC (T 5 mg/kg per day days −7 to −6, Bu 3.2 mg/kg day −3, Flu 50 mg/m2 per day days −3 and −2). RIC conditioning regimens were performed in patients who were either older than 40 years, showed a hematopoietic cell transplantation–Comorbidity Age Index >3, had previously been transplanted, or were diagnosed with Hodgkin or non-Hodgkin lymphoma or multiple myeloma.
Graft source was unmanipulated mobilized PBSCs in most patients (97 patients, 91%).
Pre- and posttransplant evaluation
Patients were stratified according to the disease risk index.14 Pretransplant comorbidities were recorded using the hematopoietic cell transplantation–Comorbidity Age Index.15 Chimerism was determined by quantitative analysis of informative microsatellite DNA polymorphisms as previously described. aGvHD was scored according to the published consensus criteria.16 cGvHD was scored according to the NIH Consensus Development Project.17
Definitions
Myeloid engraftment was defined as an absolute neutrophil count of 0.5 × 109/L or greater for 3 consecutive days. Platelet engraftment was defined as a platelet count of 20 × 09/L or higher, without transfusion support, for 3 consecutive days. Patients who survived >30 days after transplantation and who failed to achieve myeloid engraftment were considered graft failures. Diagnosis of disease recurrence was based on clinical and pathological criteria.
Statistical analysis
Quantitative variables were expressed as median and either range or interquartile range (25th and 75th percentiles). Qualitative variables were expressed as frequency and percentage. χ2 was used to test for the association between qualitative variables. Comparability of the 2 groups (PTCy and CsA-MX) for the main prognostic features was tested with Student t test. Variables, which were significantly correlated in the univariate analysis, were evaluated by logistic regression. All variables with P < .2 were included in the multivariate analysis. In order to exclude a possible center effect, the center where the patient was transplanted was included in the multivariate analysis. Primary end points were rates of aGVHD, cGVHD, and GVHD and relapse-free survival (GRFS). GRFS was defined as the first event occurring at 36 months after transplantation among aGVHD grades II to IV, moderate-severe cGVHD, relapse, or death of any cause (modified from Ruggeri et al).18 NRM, disease relapse or progression, overall survival (OS), and event-free survival (EFS) were defined as secondary end points. Relapse, toxic death, and second transplant due to graft failure were considered events. Estimates of EFS and OS were calculated using the Kaplan-Meier method, including 95% confidence interval. Cumulative incidence curves and competing risk regression were performed as alternatives to Cox regression for survival data in the presence of competing risks.19 In our case, competitor events were death and any other occurrence that prevents the appearance of the event under study. This model estimates the hazard ratio known as subdistribution hazard or subhazard ratio. For the cumulative incidence estimate of neutrophil recovery, death before day +30 was considered a competing event. For the cumulative incidence of platelet engraftment and full donor chimerism, death and retransplantation due to graft failure were considered competing events. NRM and relapse were considered competing events for each other, in addition to retransplantation for both of them. Last update of the cohort was performed in August 2018. Except for the cumulative incidence, all calculations were made with SPSS (IBM, SPSS Statistics for Windows, Version 21.0, Armonk, NY). Multivariate analysis was performed with Stata software (Version 15.1).
Results
Patients' and transplant characteristics
Between April 2014 and December 2017, 57 consecutive adult patients diagnosed with hematological malignancies were transplanted with a MSD using PTCy followed by additional immunosuppression as GVHD in 2 Spanish centers (19 from center A and 38 from center B). The historical group with CsA-MTX included 50 consecutive patients from one of the centers (center A) transplanted between March 2010 and November 2015. Baseline characteristics of the patients are summarized in Table 1.
Characteristics of patients and transplants
| . | CsA-MTX (N = 50) . | PTCy (N = 57) . | P . |
|---|---|---|---|
| Age, median (range), y | 49 (16-65) | 51 (18-67) | .877 |
| Male sex, n (%) | 34 (68) | 37 (65) | .737 |
| Diagnosis, n (%) | .402 | ||
| AML and MDS | 25 (50) | 26 (46) | |
| ALL | 11 (22) | 10 (18) | |
| NHL and CLL | 9 (18) | 11 (18) | |
| Others | 5 (10) | 10 (18) | |
| Disease risk index, n (%) | .356 | ||
| Low | 2 (4) | 3 (5) | |
| Intermediate | 35 (70) | 43 (75) | |
| High/very high | 13 (26) | 11 (20) | |
| Pretransplant status, n (%) | .602 | ||
| Complete response | 31 (62) | 36 (63) | |
| Partial response or active disease | 19 (38) | 21 (37) | |
| Donor/recipient sex, female/male, n (%) | 15 (30) | 17 (30) | .984 |
| Stem cell source, n (%) | .077 | ||
| BM | 2 (4) | 8 (14) | |
| Peripheral blood | 48 (96) | 49 (86) | |
| Graft composition, median (range) | |||
| CD34+, ×106/kg | 5.2 (4-6) | 4.4 (3-6.5) | .057 |
| TNC, ×108/kg | 9.5 (7-12) | 7.4 (5-11) | .108 |
| Conditioning regimen, n (%) | .015 | ||
| Reduced intensity | 18 (36) | 34 (60) | |
| FluMel | 11 (61) | 4 (12) | |
| Bu-Flu | 6 (33) | 6 (18) | |
| TBF-RIC | 0 (0) | 21 (65) | |
| Myeloablative | 32 (64) | 23 (40) | |
| Bu-Flu | 27 (84) | 11 (48) | |
| TBI-Cy | 4 (13) | 0 (0) | |
| TBF-MAC | 0 (0) | 12 (52) | |
| GVHD prophylaxis, n (%) | — | ||
| PTCY +3, +5, and CsA day 0 | 0 (0) | 38 (65) | — |
| PTCy +3, +4, and CsA + MMF day 5 | 0 (0) | 19 (35) | — |
| CsA day −1 and MTX +1, +3, +6 | 22 (44) | 0 (0) | — |
| CsA day −1 and MTX +1, +3, +6, +11 | 28 (56) | 0 (0) | — |
| . | CsA-MTX (N = 50) . | PTCy (N = 57) . | P . |
|---|---|---|---|
| Age, median (range), y | 49 (16-65) | 51 (18-67) | .877 |
| Male sex, n (%) | 34 (68) | 37 (65) | .737 |
| Diagnosis, n (%) | .402 | ||
| AML and MDS | 25 (50) | 26 (46) | |
| ALL | 11 (22) | 10 (18) | |
| NHL and CLL | 9 (18) | 11 (18) | |
| Others | 5 (10) | 10 (18) | |
| Disease risk index, n (%) | .356 | ||
| Low | 2 (4) | 3 (5) | |
| Intermediate | 35 (70) | 43 (75) | |
| High/very high | 13 (26) | 11 (20) | |
| Pretransplant status, n (%) | .602 | ||
| Complete response | 31 (62) | 36 (63) | |
| Partial response or active disease | 19 (38) | 21 (37) | |
| Donor/recipient sex, female/male, n (%) | 15 (30) | 17 (30) | .984 |
| Stem cell source, n (%) | .077 | ||
| BM | 2 (4) | 8 (14) | |
| Peripheral blood | 48 (96) | 49 (86) | |
| Graft composition, median (range) | |||
| CD34+, ×106/kg | 5.2 (4-6) | 4.4 (3-6.5) | .057 |
| TNC, ×108/kg | 9.5 (7-12) | 7.4 (5-11) | .108 |
| Conditioning regimen, n (%) | .015 | ||
| Reduced intensity | 18 (36) | 34 (60) | |
| FluMel | 11 (61) | 4 (12) | |
| Bu-Flu | 6 (33) | 6 (18) | |
| TBF-RIC | 0 (0) | 21 (65) | |
| Myeloablative | 32 (64) | 23 (40) | |
| Bu-Flu | 27 (84) | 11 (48) | |
| TBI-Cy | 4 (13) | 0 (0) | |
| TBF-MAC | 0 (0) | 12 (52) | |
| GVHD prophylaxis, n (%) | — | ||
| PTCY +3, +5, and CsA day 0 | 0 (0) | 38 (65) | — |
| PTCy +3, +4, and CsA + MMF day 5 | 0 (0) | 19 (35) | — |
| CsA day −1 and MTX +1, +3, +6 | 22 (44) | 0 (0) | — |
| CsA day −1 and MTX +1, +3, +6, +11 | 28 (56) | 0 (0) | — |
ALL, acute lymphoid leukemia; AML, acute myeloid leukemia; CLL, chronic lymphatic leukemia; MDS, myelodysplastic syndrome; Mel, melphalan; NHL, non-Hodgkin lymphoma; TBI, total body irradiation; TNC, total nucleated cell count.
The median age of the total study population was 51 years. Acute myeloid and lymphoid leukemia were the most frequent transplant indication (67%). Most of the patients had intermediate (73%) and high-/very high-risk disease (22%). Baseline characteristics were comparable between groups, with the exception of conditioning regimen, with RIC regimens more frequently used in the PTCy group (60% vs 36%, P = .015). Active disease as pretransplant status (defined as stable disease, partial response, or progressive disease) was similar in both groups. Peripheral blood was the predominant graft source in both groups (91%).
Engraftment and chimerism
All patients from the group achieved myeloid engraftment; no primary graft failures were observed. Only 1 patient from the PTCy group was diagnosed with secondary graft failure due to CMV disease after initial engraftment and was treated with a second HSCT from an HLA haploidentical related donor at month +5. A total of 3 patients in the MTX-CsA group and 2 patients in the PTCy group died before day +100 due to NRM without achieving platelet engraftment.
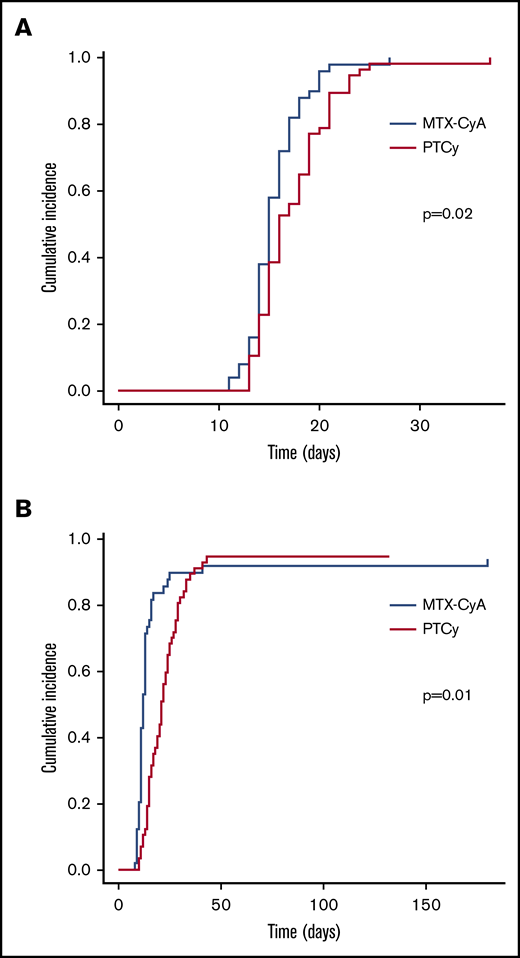
Neutrophil and platelet engraftments were significantly delayed in the PTCy group as compared with the MTX-CsA group with median days of engraftment of 15.5 (13 to 37) vs 14.5 (11 to 27) days (P = .02), and 20.5 (10 to 43) vs 11.5 (8 to 180) days (P = .02), respectively (Figure 1). Cumulative incidence of neutrophil recovery at day 28 was 100% in the MTX-CsA group compared with 98% in the PTCy group (P = .02). The day-28 cumulative incidence of platelet recovery was 95% and 79% (P = .01).
Neutrophil and platelet engraftment. (A) Neutrophil engraftment. (B) Platelet engraftment.
Neutrophil and platelet engraftment. (A) Neutrophil engraftment. (B) Platelet engraftment.
Median time to full-donor chimerism achievement in peripheral blood was 29.5 days (12 to 240) in the MTX-CsA group and 29.5 days (14 to 217) in the PTCy group. Full-donor chimerism was achieved in 37/46 (80%) evaluable patients by day +60 in the MTX-CsA and 47/56 (84%) in the PTCy group.
OS and EFS
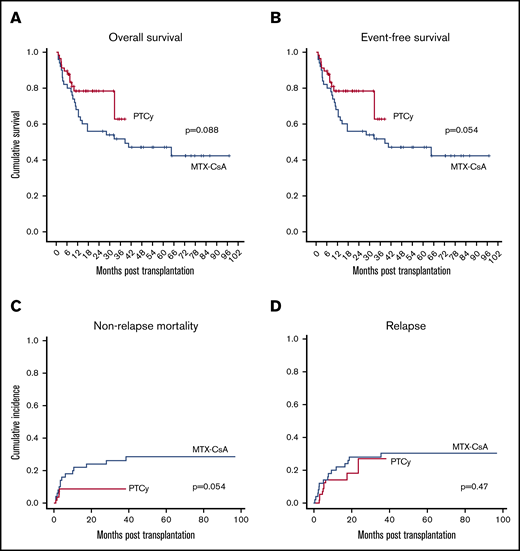
After a median follow-up of 60 months for the MTX-CsA group and 15 months for the PTCy group, 2-year OS and EFS were higher in the PTCy group, although not statistically significant: 56% (42 to 70) and 78% (67 to 90) (P = .088), and 48% (34 to 62) and 62.5% (42.5 to 82.5) (P = .054), respectively (Figure 2A-B).
Survival, mortality and relapse data. OS (A), EFS (B), NRM (C), and relapse incidence (D).
Survival, mortality and relapse data. OS (A), EFS (B), NRM (C), and relapse incidence (D).
GVHD and GRFS
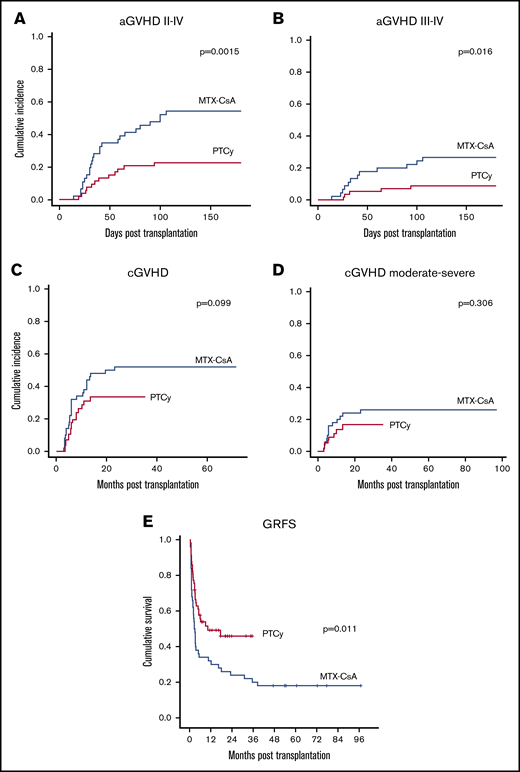
Cumulative incidence at 100 days of aGVHD grade II to IV (52.2% vs 22.6%, P = .0015) (Figure 3A), and III to IV (24.4% vs 8.8%, P = .016) (Figure 3B) were significantly higher in the MTX-CsA group. In the MTX-CsA group, 25 patients experienced grade II to IV aGVHD, 13 meeting criteria for grade II aGVHD (8 of them with gastrointestinal, 8 with cutaneous, and 6 with hepatic involvement). After systemic steroids, 6 of 13 achieved complete response and 7 of 13 achieved partial response; none of these patients died due to GVHD. Twelve out of 25 patients developed grade III to IV aGVHD (10 with gastrointestinal, 11 cutaneous, and 7 hepatic involvement). After initial therapy with steroids, 11 of them required a combination of second-line treatments (6 mesenchymal stem cells, 5 antitumor necrosis factor, 4 extracorporeal photopheresis, 1 antithymocyte globulin). The median number of treatments added to steroids for these patients was 3. Four out of 11 patients were refractory to treatment and died due to aGVHD; 3 patients who achieved either partial or complete response died due to infections in the setting of the immunosuppressive treatment. Within the PTCy cohort, 7 patients developed grade II aGVHD (4 cutaneous, 3 gastrointestinal, and 1 hepatic involvement). All patients achieved partial (2/7) or complete response (5/7). Five patients developed grade III to IV aGVHD (3 gastrointestinal, 1 pulmonary, 1 cutaneous, and 1 hepatic involvement). Two out of 5 responded to steroid therapy, whereas 3 required second-line treatments, with a median number of drugs added to steroid treatment in these patients being 1.5. Two of the patients who did not respond to the initial treatment died due to aGVHD. There were no patients meeting criteria of late onset aGVHD in either group. Multivariate analysis, including age, sex, diagnosis, comorbidity, transplant center, pretransplant status, stem cell source, disease risk index, and intensity of conditioning regimen, showed that GVHD prophylaxis regimen with PTCy and the use of BM as graft source (P = .006 and P < .001, respectively) were protective factors for the development of aGVHD grade II to IV. The use of peripheral blood as source was also identified as the unique independent risk factor for the development of aGVHD grade III to IV (P < .001).
GVHD cumulative incidence and survival data. (A) aGVHD grades II to IV cumulative incidence. (B) aGVHD grades III to IV cumulative incidence. (C) cGVHD cumulative incidence. (D) Chronic moderate to severe GVHD cumulative incidence. (E) GVHD-free and GRFS.
GVHD cumulative incidence and survival data. (A) aGVHD grades II to IV cumulative incidence. (B) aGVHD grades III to IV cumulative incidence. (C) cGVHD cumulative incidence. (D) Chronic moderate to severe GVHD cumulative incidence. (E) GVHD-free and GRFS.
Cumulative incidence of cGVHD showed a higher trend in the MTX-CsA group (52% vs 34%, P = .099) (Figure 3C). The 2-year cumulative incidence of moderate and severe cGVHD was higher in the MTX-CsA cohort (26% vs 16.7%, P = .306) (Figure 3D), although not statistically significant. Twenty-seven patients in the MTX-CsA group developed cGVHD: 14 of them experienced mild affectation, mostly with cutaneous-mucosal involvement, most of them achieving partial response with topical treatment or reintroduction of the immunosuppressive regimen with CsA. Thirteen patients developed moderate to severe cGVHD, most of them with sclerotic skin features and/or other organ involvement (hepatic, gastrointestinal tract), requiring a median of 3 treatments to achieve response, including steroids, extracorporeal photopheresis, CNIs, antitumor necrosis factor, and mesenchymal stem cells. Only 2 patients in this group achieved complete response, and 4 patients died due to GVHD or infections in the setting of immunosuppression. Within the PTCy group, 17 patients developed cGVHD, 9 of them with local manifestations. Eight patients developed moderate-severe cGVHD, most of them with hepatic involvement; none of the patients developed sclerotic morphea-like skin features or fascia-muscle-joint involvement. There were no deaths due to cGVHD in this group. Median number of treatments required was 2. None of the variables included in the multivariate analysis, including group of GVHD prophylaxis regimen (P = .124), were identified as independent factors for the development of cGVHD. However, intensity of the conditioning regimen was an independent factor for the development moderate-severe cGVHD (P = .013).
The composite endpoint of GRFS at 2 years was significantly higher in the PTCy group (48% vs 24%, P = .011) (Figure 3E). In the multivariate analysis, PTCy was identified as the sole independent favorable factor for this endpoint (P = .035).
Considering patients alive after 1-year follow-up and excluding those who had received donor lymphocyte infusions and/or had withdrawn immunosuppression due to relapse, 80% of evaluable patients in the PTCy group and 51% in the MTX-CsA cohort were off immunosuppressive therapy at 1 year (P < .05).
Toxicity and NRM
No statistically significant differences were found regarding the 100-day incidence of grade II to IV oral or gastrointestinal mucositis (60% vs 53%, P = .250), grade II to IV hepatotoxicity (14% vs 5%, P = .123), grade II to IV hemorrhagic cystitis (8% vs 14%, P = .526), and CMV reactivation (40% vs 49%, P = .346) between the MTX-CsA and the PTCy groups, respectively. There were no episodes of EBV reactivations or PTLD in either of the 2 groups. The incidence of sinusoidal obstruction syndrome (SOS) was very low, with only 2 cases in the MTX-CsA group. None of the patients in the PTCy group developed SOS.
Cumulative incidence of NRM at 2 years was higher in the MTX-CsA group (24% vs 8.8%, P = .054) (Figure 2C). NRM in the MTX-CsA group (including events developed after the first 2 years of follow-up) was due to GVHD in 10 out of 14 patients, SOS in 1 patient, and septicemia in 3 patients.
In the PTCy group, causes of NRM were aGVHD with idiopathic pneumonia syndrome in 1 case out of 5, infection in 3, and posterior reverse encephalopathy syndrome in 1 case. In the multivariate analysis, PTCy showed a trend to be a protector factor for NRM (P = .068).
Relapse
No statistically significant differences were observed in the 2-year cumulative incidence of relapse (27% vs 28%, P = .47) (Figure 2D). Among the 15 patients who experienced relapse in the MTX-CsA group, 11 relapses occurred within first 12 months post-HSCT. Six of the patients received donor-lymphocyte infusion, and 2 underwent a second transplant after achieving a subsequent complete response. In the PTCy group, 10 patients relapsed, 8 of them in the first 12 months post-HSCT. Similarly, 6 of them received donor-lymphocyte infusion, and 3 received a second transplant. None of the variables included in the multivariate analysis were identified as independent factors for relapse.
Discussion
HLA-identical sibling donor remains the first option for patients in need of an allogeneic HSCT. In this setting, the combination of MTX and a CNI has been the standard and most extended GVHD prophylaxis used since the 1980s. However, rates of aGVHD and cGVHD remain significant with this strategy, especially using PBSCs, accounting not only for the main cause of NRM after HSCT from MSD but also for the high comorbidity burden for long-term survivors derived from cGVHD.20 In an attempt to reduce the incidence of significant GVHD and NRM, Luznik et al have pioneered the use of PTCy as sole prophylaxis in the HLA-identical donor setting with BM stem cells with promising results.6 Furthermore, PTCy has been shown to decrease the global immunosuppressive burden experienced by patients undergoing HLA-matched HCST with BM stem cells.21 PTCy has also been introduced in the PB stem cells setting with initial discouraging results when used as sole prophylaxis due to high rates of severe GVHD,8,9 overcame by the sum of additional immunosuppressive drugs.10 However, comparative studies with classic prophylaxis strategies confined to MSD are scarce.
In this retrospective comparative study of MSD HSCT, with PBSCs used mostly as graft source, PTCy combined with additional immunosuppression was shown to significantly decrease the rate of aGVHD grade II to IV and III to IV compared with the classical strategy using MTX and CsA together with a significant superior GRFS. These results are in line with those observed in previous retrospective comparative studies, including MSD.22,23 However, these previous reports included matched unrelated donors in the analysis, MSD being the minority of the study population. Although PTCy patients received reduced intensity conditioning regimen in a higher proportion compared with the MTX-CsA cohort, multivariate analysis showed GVHD prophylaxis regimen and the use of PBSCs as sole independent factors for the development of aGVHD. PTCy prophylaxis also showed lower rates of cGVHD (52% vs 34%) and moderate-severe cGVHD (26% vs 16.7%), although not statistically significant due to relatively small sample size. Of note, none of the patients who developed significant cGVHD in the PTCy group showed sclerotic forms or fascia-joint involvement, with hepatic involvement the most frequent feature. For those patients developing GVHD, a higher amount of additional immunosuppressive drugs was required and used in the MTX-CsA group compared with the PTCy group. Furthermore, a significantly higher proportion of evaluable patients in the PTCy group (80%) were off immunosuppressive therapy at 1 year compared with the MTX-CsA cohort (51%). Finally, lower rates of significant GVHD in the PTCy group lead to less mortality associated with GVHD. In fact, most of the causes of nonrelapse-related deaths in the PTCy group were infectious complications, whereas the leading cause of death in the MTX-CsA group was GVHD.
The incidence of cGVHD in the PTCy group was higher than that reported using BM as stem cell source and PTCy alone as GVHD prophylaxis.5 The use of MAC regimen in 40% of the patients and, more importantly, the use of PBSCs in 86% of the cases might account for higher rates of cGVHD. The addition of immunosuppressive drugs has been shown to decrease the rate of significant GVHD in the PBSC setting and PTCy, and the use of 2 drugs might further improve results compared with the addition of only 1 drug.11 Only prospective trials will draw definite conclusions. In the absence of randomized studies, and the published experience reported up to date, PBSCs seem to offer higher rates of cGVHD compared with BM even with the use of additional immunosuppressive drugs. Until such studies develop or modified strategies take place in the PBSC transplant setting, stem cells source should be taken into consideration if possible, for patients with high risk of GVHD development and older patients.
The majority (65%) of the patients in the PTCy cohort received a CNI on day 0 (ie, before PTCy). Potentially, this could dampen the T-cell proliferation on encountering the recipient antigens, reducing efficacy of PTCy.24 However, in the HLA-haploidentical donor setting, the start of CNI and MMF previous to the infusion of Cy has been shown in a single experience to provide low rates of aGVHD and cGVHD, overall lower than those obtained with the original Johns Hopkins approach, as well as NRM, while preserving disease control.13 A reduced impact of PTCy on less proliferating alloreactive T lymphocytes and/or the effect of regulatory T cells in this setting may explain these intriguing results.25 In the present analysis in MSD transplants, rates of aGVHD after PTCy were lower than those obtained with MTX-CsA, and whether the use of immunosuppression before the administration of Cy could provide even lower rates of GVHD deserves further investigation.
Although the addition of CNI and MMF to PTCy could theoretically be detrimental for the development of adequate graft-versus-leukemia effect, no significant impact was observed on relapse rates compared with MTX-CsA. In addition, none of the factors included in the multivariate analysis, including GVHD prophylaxis, graft source, and intensity of conditioning regimen, showed an impact on relapse rates. However, shorter median follow-up of the PTCy warrants further analysis.
On the other hand, the use of PTCy was associated with prolonged time for neutrophil engraftment compared with MTX-CsA. However, rates of achievement of full donor chimerism were similar, and furthermore, no cases of primary graft failure occurred in either cohort. Organ toxicity and infectious complications were not different in both groups, suggesting that PTCy combined with additional IS does not prevent an adequate immune reconstitution. In the setting of HLA-haploidentical HSCT, PTCy has been shown to be more toxic to naive T cells, responsible for severe GVHD, than to memory T cells and regulatory T cells, which in part leads to an effective prevention of severe GVHD and, at the same time, secures an adequate antitumor effect together with sufficient immune reconstitution.26,27 Similarily, in HLA-matched donor transplants with BM as graft source, the use of PTCy as sole GVHD prophylaxis provided prompt immune reconstitution and a low incidence of opportunistic infections, suggesting similar mechanisms of high-dose PTCy in the HLA-haploidentical donor setting.26 Further analysis of posttransplant immune reconstitution comparing MTX plus CNI and PTCy in HLA-matched donor HSCT will deepen the understanding of this unique effect of selective in vivo lymphodepletion.
Limitations of this study include its retrospective nature and the limited number of patients analyzed. Inherent to all multicentric retrospective studies, potential discordances in diagnosis and grading of GVHD have to be taken into account. However, in the present study, a possible center effect has been excluded in the multivariate analysis. Also, the study was underpowered to draw definitive conclusions for some of the endpoints due to the retrospective nature of the analysis and the relatively small sample analyzed. All of which warrants prospective randomized studies in this field. Nevertheless, the present data, in aggregate, do favor PTCy and are in keeping with previous reports.
Of note, the definition of GRFS used in the present study included grade II aGVHD, which is stricter with respect to previous definitions.18 Efforts should be directed toward the avoidance of all forms of GVHD that require systemic immunosuppression, which compromises survival and consumes a great amount of resources, sparing an effective graft-versus-leukemia effect. With this objective, the use of PTCy as GVHD prophylaxis in PBSC HLA-MSD HSCT provides better GRFS rates that could translate favorably on the quality of life of long-term survivors.
In conclusion, in our experience, GVHD prophylaxis based on PTCy and additional immunosuppression improves the results of PBSC HLA-identical sibling HSCT. Future studies should be aimed to improve the selection and duration of additional IS in order to optimize the strategy depending on indication, donor characteristics, and graft source.
Acknowledgments
The authors thank the staff and nurses of all the hematology and transplant units for their care and contributions to making this work possible. They thank Jose Maria Bellón from the Instituto de Investigación Sanitaria Gregorio Marañon for data analysis.
This work was partially supported by the Ministry of Economy and Competitiveness ISCIII-FIS grants PI08/1463, PI11/00708, PI14/01731, and RD12/0036/0061, cofinanced by European Regional Development Fund (Fondo Europeo de Desarrollo Regional) funds from the European Commission, “A way of making Europe,” as well as grants from the Fundación LAIR, Asociación Madrileña de Hematología y Hemoterapia, Asociación Española Contra el Cáncer, and Fundación Mutua Madrileña.
Authorship
Contribution: M.K., R.B., M.J.P.-C., and P.B. conceived and designed the study; M.K., R.B., M.J.P.-C., A.I.G.-M., A.G.S., P.B., L.S., N.D., C.M., D.S., C.M.-L., I.B., J.A., and J.L.D.-M. provided the study materials or patients; M.K., R.B., M.J.P.C., and P.B. collected and assembled the data; M.K., R.B., M.J.P.-C., A.I.G.-M., A.G.S., P.B., L.S., N.D., C.M., D.S., C.M.-L., I.B., J.A., and J.L.D.-M. analyzed and interpreted the data; M.K., R.B., and M.J.P.-C. wrote the manuscript; and M.K., R.B., M.J.P.-C., A.I.G.-M., A.G.S., P.B., L.S., N.D., C.M., D.S., C.M.-L., I.B., J.A., and J.L.D.-M. had final approval of the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Mi Kwon, Department of Hematology, Hospital General Universitario Gregorio Marañon, Doctor Esquerdo 46, 28007 Madrid, Spain; e-mail: mi.kwon@salud.madrid.org.
References
Author notes
M.K. and B.R. contributed equally to this study.