Issue Archive
Table of Contents
EDITORIAL
Introduction to a How I Treat series on acute lymphoblastic leukemia
Edited by Associate Editor Hervé Dombret, this How I Treat series highlights the clinical approach to high-risk subgroups of acute lymphoblastic leukemia (ALL). These include adult Philadelphia chromosome (Ph)–positive ALL, Ph-like ALL, infant acute ALL, early T-cell precursor ALL, Ph-negative ALL in older patients, and postimmunotherapy relapsed B-cell ALL. Using illustrative cases, the authors outline the approach to these patients, with recommendations with regard to targeted therapies, novel therapeutic approaches, and the changing role of allogeneic stem cell transplantation.
BLOOD COMMENTARIES
HOW I TREAT SERIES
How I treat adult Ph+ ALL
Edited by Associate Editor Hervé Dombret, this How I Treat series highlights the clinical approach to high-risk subgroups of acute lymphoblastic leukemia (ALL). These include adult Philadelphia chromosome (Ph)–positive ALL, Ph-like ALL, infant acute ALL, early T-cell precursor ALL, Ph-negative ALL in older patients, and postimmunotherapy relapsed B-cell ALL. Using illustrative cases, the authors outline the approach to these patients, with recommendations with regard to targeted therapies, novel therapeutic approaches, and the changing role of allogeneic stem cell transplantation.
How I treat Philadelphia chromosome–like acute lymphoblastic leukemia in children, adolescents, and young adults
Edited by Associate Editor Hervé Dombret, this How I Treat series highlights the clinical approach to high-risk subgroups of acute lymphoblastic leukemia (ALL). These include adult Philadelphia chromosome (Ph)–positive ALL, Ph-like ALL, infant acute ALL, early T-cell precursor ALL, Ph-negative ALL in older patients, and postimmunotherapy relapsed B-cell ALL. Using illustrative cases, the authors outline the approach to these patients, with recommendations with regard to targeted therapies, novel therapeutic approaches, and the changing role of allogeneic stem cell transplantation.
How I treat infant acute lymphoblastic leukemia
Edited by Associate Editor Hervé Dombret, this How I Treat series highlights the clinical approach to high-risk subgroups of acute lymphoblastic leukemia (ALL). These include adult Philadelphia chromosome (Ph)–positive ALL, Ph-like ALL, infant acute ALL, early T-cell precursor ALL, Ph-negative ALL in older patients, and postimmunotherapy relapsed B-cell ALL. Using illustrative cases, the authors outline the approach to these patients, with recommendations with regard to targeted therapies, novel therapeutic approaches, and the changing role of allogeneic stem cell transplantation.
How I treat ETP-ALL in children
Edited by Associate Editor Hervé Dombret, this How I Treat series highlights the clinical approach to high-risk subgroups of acute lymphoblastic leukemia (ALL). These include adult Philadelphia chromosome (Ph)–positive ALL, Ph-like ALL, infant acute ALL, early T-cell precursor ALL, Ph-negative ALL in older patients, and postimmunotherapy relapsed B-cell ALL. Using illustrative cases, the authors outline the approach to these patients, with recommendations with regard to targeted therapies, novel therapeutic approaches, and the changing role of allogeneic stem cell transplantation.
How I treat older patients with Ph/BCR-ABL–negative acute lymphoblastic leukemia
Edited by Associate Editor Hervé Dombret, this How I Treat series highlights the clinical approach to high-risk subgroups of acute lymphoblastic leukemia (ALL). These include adult Philadelphia chromosome (Ph)–positive ALL, Ph-like ALL, infant acute ALL, early T-cell precursor ALL, Ph-negative ALL in older patients, and postimmunotherapy relapsed B-cell ALL. Using illustrative cases, the authors outline the approach to these patients, with recommendations with regard to targeted therapies, novel therapeutic approaches, and the changing role of allogeneic stem cell transplantation.
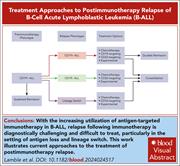
How I treat postimmunotherapy relapsed B-ALL
Edited by Associate Editor Hervé Dombret, this How I Treat series highlights the clinical approach to high-risk subgroups of acute lymphoblastic leukemia (ALL). These include adult Philadelphia chromosome (Ph)–positive ALL, Ph-like ALL, infant acute ALL, early T-cell precursor ALL, Ph-negative ALL in older patients, and postimmunotherapy relapsed B-cell ALL. Using illustrative cases, the authors outline the approach to these patients, with recommendations with regard to targeted therapies, novel therapeutic approaches, and the changing role of allogeneic stem cell transplantation.
CLINICAL TRIALS AND OBSERVATIONS
Dexamethasone dose intensity does not impact outcomes in newly diagnosed multiple myeloma: a secondary SWOG analysis
Clinical Trials & Observations
Dexamethasone (Dex) has been a key component of induction therapy for newly diagnosed multiple myeloma (NDMM) for decades but is commonly associated with toxicity. Banerjee et al performed a secondary analysis of 2 large SWOG studies to determine the impact of toxicity-induced reductions in Dex dosing on progression-free survival (PFS) and overall survival (OS) in NDMM. A surprising 69% of patients required dose reduction of Dex due to grade 3 or greater toxicity, but this had no impact on PFS or OS, suggesting that in the era of modern treatment regimens, studies should focus on Dex dose reduction.
Safety and efficacy of standard-of-care ciltacabtagene autoleucel for relapsed/refractory multiple myeloma
Clinical Trials & Observations
Ciltacabtagene autoleucel was approved in 2022 for patients with relapsed/refractory multiple myeloma, based on the results of the CARTITUDE-1 trial. Sidana and colleagues evaluated the results of 255 “real world” patients treated at 16 US academic medical centers, of whom 54% would not have been eligible of the CARTITUDE-1 trial, mostly because of older age and prior exposure to B-cell maturation antigen–targeted therapy. These patients have a favorable efficacy profile despite not fulfilling clinical trial eligibility criteria.
GENE THERAPY
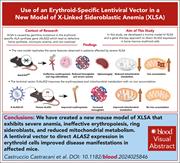
An erythroid-specific lentiviral vector improves anemia and iron metabolism in a new model of XLSA
X-linked sideroblastic anemia (XLSA) is caused by mutations in ALAS2, the enzyme responsible for the first step in heme synthesis. Castruccio Castracani et al report results using a conditional knockout model of XLSA that manifests severe anemia with ineffective erythropoiesis linked to mitochondrial dysfunction, splenomegaly, low hepcidin, systemic iron accumulation, and marrow ring sideroblasts. The authors developed a lentiviral vector that directs ALAS2 expression in erythroid cells that improves the phenotype, suggesting a potential curative approach for patients with XLSA who respond poorly to pyridoxine.
HEMATOPOIESIS AND STEM CELLS
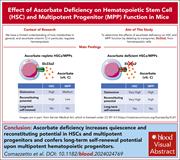
Ascorbate deficiency increases quiescence and self-renewal in hematopoietic stem cells and multipotent progenitors
Ascorbate (vitamin C) limits hematopoietic stem cell (HSC) function and suppresses leukemia by promoting the function of the Tet2 tumor suppressor. Comazzetto and colleagues deleted the ascorbate transporter from HSCs in mice and demonstrated that intracellular ascorbate deficiency in HSCs and multipotent progenitors (MPPs) increases quiescence and self-renewal in both HSCs and MPPs.
THROMBOSIS AND HEMOSTASIS
Cost-effectiveness of iptacopan for paroxysmal nocturnal hemoglobinuria
Iptacopan, an oral factor B inhibitor, has been approved by the US Food and Drug Administration for the treatment of paroxysmal nocturnal hemoglobinuria. Ito et al examined the cost-effectiveness of iptacopan over the C5 inhibitors eculizumab and ravulizumab, both parenteral agents that are limited by persistent extravascular hemolysis and anemia. The authors confirm that iptacopan monotherapy can be cost-effective in patients with inadequate response to C5 inhibitors based on monetary benefit and time saved for patients and nurses.
BLOOD WORK
-
Cover Image
Cover Image
![issue cover]()
Perls iron staining of isolated polychromatic erythroid cells. The novel X-linked sideroblastic anemia (XLSA) model with a complete deletion of Alas2 (Alas2-KOBM) shows ring sideroblasts, a typical clinical feature of patients affected by XLSA. See the article by Castruccio Castracani et al on page 98.
- PDF Icon Front MatterFront Matter
- PDF Icon Table of ContentsTable of Contents
- PDF Icon Editorial BoardEditorial Board
Advertisement intended for health care professionals
Email alerts
Advertisement intended for health care professionals