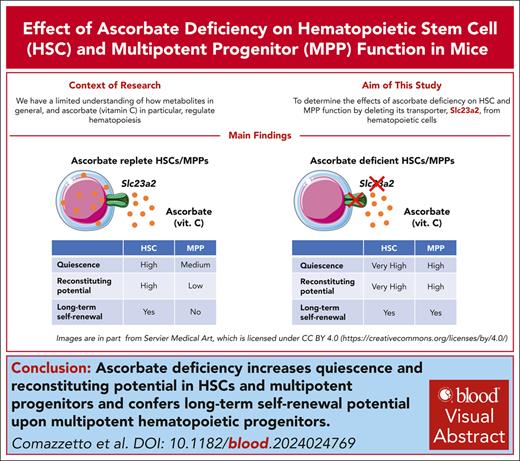
Key Points
Deletion of the ascorbate transporter, Slc23a2, increases quiescence and self-renewal potential in HSCs and multipotent progenitors.
Ascorbate deficiency is sufficient to confer long-term self-renewal potential on MPPs.
Visual Abstract
Ascorbate (vitamin C) limits hematopoietic stem cell (HSC) function and suppresses leukemia development, partly by promoting the function of the Tet2 tumor suppressor. In humans, ascorbate is obtained from the diet, whereas in mice, it is synthesized in the liver. In this study, we show that deletion of the Slc23a2 ascorbate transporter from hematopoietic cells depleted ascorbate to undetectable levels in HSCs and multipotent hematopoietic progenitors (MPPs) without altering the plasma ascorbate levels. Slc23a2 deficiency increased HSC reconstituting potential and self-renewal potential upon transplantation into irradiated mice. Slc23a2 deficiency also increased the reconstituting and self-renewal potentials of MPPs, conferring the ability to reconstitute irradiated mice long term. Slc23a2-deficient HSCs and MPPs divided much less frequently than control HSCs and MPPs. Increased self-renewal and reconstituting potential were observed particularly in quiescent Slc23a2-deficient HSCs and MPPs. The effect of Slc23a2 deficiency on MPP self-renewal was not mediated by reduced Tet2 function. Ascorbate thus regulates quiescence and restricts self-renewal potential in HSCs and MPPs such that ascorbate deficiency confers MPPs with long-term self-renewal potential.
Introduction
The daily production of blood cells in mammals is sustained by hematopoietic stem cells (HSCs), multipotent hematopoietic progenitors (MPPs), and restricted hematopoietic progenitors that reside in the bone marrow. HSCs have long-term self-renewal potential and give long-term multilineage reconstitution upon transplantation into irradiated mice.1-3 MPPs persist and contribute to hematopoiesis over long periods of time in normal adult mice,4-8 but when small numbers of MPPs are transplanted into irradiated mice they exhibit limited self-renewal potential and only transiently reconstitute.9-11 HSCs seem to undergo a limited number of cell divisions before being fated to differentiate because quiescent HSCs have more reconstituting potential and more self-renewal potential than dividing HSCs.12,13 Adult HSCs that have undergone multiple rounds of cell division have little reconstituting potential.14,15
A few mechanisms that limit the self-renewal potential of MPPs have been identified. Deficiency in Jumonji or AT-rich interactive domain-containing protein 2 (Jarid2), a transcriptional repressor, confers long-term reconstituting potential upon MPPs, partly by increasing the expression of Runx1t1 and Mycn.16 Deficiency in Trp53 and Cdkn2a increases the reconstituting potential of HSCs and confers long-term reconstituting potential onto MPPs.17
Metabolomic analysis showed that HSCs, MPPs, and restricted hematopoietic progenitors are metabolically distinct.18-21 Ascorbate (vitamin C) is obtained from the diet in humans but is synthesized in the liver of mice.22 Ascorbate uptake by hematopoietic cells is mediated by the Slc23a2 transporter,18,23 which is highly selective for ascorbate.24-27 Ascorbate levels are high in HSCs and MPPs but decline with differentiation.18 In a previous study, we systemically depleted ascorbate in gulonolactone oxidase (Gulo)-deficient mice, which lack the ability to synthesize ascorbate.18 Systemic ascorbate depletion increases the reconstituting potential of HSCs and promotes the development of leukemia, largely by reducing the function of Tet2, a tumor suppressor that demethylates DNA.18 However, this study did not assess the effect of complete ascorbate deficiency in hematopoietic cells because we did not have a way of doing this in adult mice without causing the mice to develop scurvy. Ascorbate is also necessary for T cell, plasma B cell, and erythrocyte differentiation.18,23,28-30
In this study, we tested whether ascorbate deficiency in HSCs and MPPs altered their function in the absence of systemic changes in ascorbate levels. We eliminated detectable ascorbate from HSCs and MPPs without altering systemic ascorbate levels by deleting the Slc23a2 ascorbate transporter from hematopoietic cells. These mice did not develop scurvy; they had normal Slc23a2 function in nonhematopoietic cells and normal plasma ascorbate levels. We found that ascorbate promotes cell division in HSCs and MPPs under steady-state conditions and negatively regulates their self-renewal potential.
Methods
Mice
Slc23a2 floxed mice (Slc23a2FL) were generated in a C57Bl/Ka background by introducing LoxP sites around exon 5 (which encodes part of the ascorbate transporter domain) using CRISPR-Cas9 (supplemental Figure 1A-C, available on the Blood website). Cre-mediated recombination of this allele deletes exon 5, which leads to a frameshift that eliminates Slc23a2-mediated ascorbate uptake. Slc23a2FL/+ mice were backcrossed at least 3 times onto a C57Bl/Ka background. We also employed Mx1-Cre mice31 (RRID:IMSR_JAX:003556), Scl-CreER mice (RRID:IMSR_JAX:037466),32Tet2FL mice33 (RRID:IMSR_JAX:017573), Gulo− mice,34 and Col1a1-H2B-GFP;Rosa26-M2-rtTA mice15 (RRID:IMSR_JAX:016836). All mice were maintained on a C57BL/Ka background. We used 8- to 12-week-old littermates or age-matched mice in all experiments. To induce Cre recombinase expression, Mx1-Cre mice were given 3 intraperitoneal injections of 40 μg polyinosinic-polycytidilic acid (poly I:C) (GE Healthcare) dissolved in phosphate-buffered saline over 5 days. Mice were housed in Association for Assessment and Accreditation of Laboratory Animal Care (AAALAC)-accredited, specific-pathogen-free animal care facilities at the University of Texas Southwestern Medical Center. All procedures were approved by the University of Texas Southwestern Medical Center Institutional Animal Care and Use Committee.
Flow cytometric analysis and sorting of hematopoietic cells
The bone marrow was flushed from tibias and femurs using staining medium (Hanks Buffered Saline Solution supplemented with 2% bovine serum) and was dissociated into a single-cell suspension by gently triturating with a 23-gauge needle and then filtering through a 40 μm cell strainer. The cells were counted and subsequently stained with antibodies at 4°C for 30 minutes (except for anti-CD34, which was incubated for 90 minutes). For staining of HSCs and hematopoietic progenitors, cells were stained with antibodies against lineage markers (FITC- or APC-conjugated antibodies against CD2, CD3, CD5, CD8a, B220, Ter119, and Gr1), washed with staining medium, and then resuspended in fluorophore-conjugated antibodies against c-kit, Sca1, CD150, CD48, CD16/32, Flt3, IL7Rα, CD105, and CD41. For the analysis of lymphoid and myeloid cells, the cells were stained using fluorophore-conjugated antibodies against Mac-1, B220, CD3, CD115, Ly6c, and Ly6g. Dead cells were identified and gated out of all analyses by staining with DAPI (4′,6-diamidino-2-phenylindole) or with propidium iodide after antibody staining. Cells were analyzed using a FACSAria II (BD Biosciences), a FACSAria Fusion (BD Biosciences), or a FACS Lyric cytometer.
To sort HSCs, MPPs, hematopoietic progenitor cell (HPC)1 cells, and HPC2 cells, tibias, femurs, pelvises, and vertebrae were crushed using a mortar and pestle. Cells were resuspended in staining medium and filtered through a 40 μm cell strainer. The cells were stained with APC-efluo780 conjugated anti–c-kit antibody and c-kit+ cells were enriched using anti-APC paramagnetic microbeads (Miltenyi Biotec). The cells were stained with fluorophore-conjugated antibodies against lineage markers (CD2, CD3, CD5, CD8a, B220, Ter119, and Gr1), Sca1, CD150, and CD48. The cells were isolated using 2 successive rounds of sorting to ensure purity using a FACSAria II cytometer.
Transplantation assays
Recipient (CD45.1/CD45.2) mice were irradiated using an XRAD 320 X-ray irradiator (Precision X-Ray Inc) with 2 doses of 540 rad at least 4 hours apart. For whole bone marrow transplantation, 500 000 unfractionated bone marrow cells from donor (CD45.2) mice and 2 million unfractionated bone marrow cells from competitor (CD45.1) mice were mixed and injected intravenously through the retro-orbital venous sinus. For HSC, MPP, HPC1, and HPC2 cell transplants, the number of cells indicated in each experiment were mixed with 350 000 unfractionated bone marrow cells from competitor (CD45.1) mice and injected intravenously into the retro-orbital venous sinus. For secondary transplantation assays, 5 million unfractionated bone marrow cells from primary recipient mice were injected intravenously into irradiated secondary recipient mice. Every 4 weeks until at least 16 to 20 weeks after transplantation, blood was collected from the tail vein and subjected to ammonium chloride–potassium chloride red cell lysis. Cells were then stained with antibodies against CD45.1, CD45.2, Mac-1, Gr1, B220, and CD3 to evaluate the levels of donor cell engraftment in the myeloid, B-, and T-cell lineages. Recipient mice were considered long-term reconstituted if donor myeloid, B, and T cells were present for at least 16 weeks after transplantation (>0.5% of myeloid, B, and T cells were donor derived). Recipient mice were considered short-term multilineage reconstituted if donor myeloid, B, and/or T cells were detected in the recipient mice, but donor myeloid cells became undetectable before 16 weeks after transplantation.
Results
Ascorbate deficiency increases reconstituting potential in transplantation assays
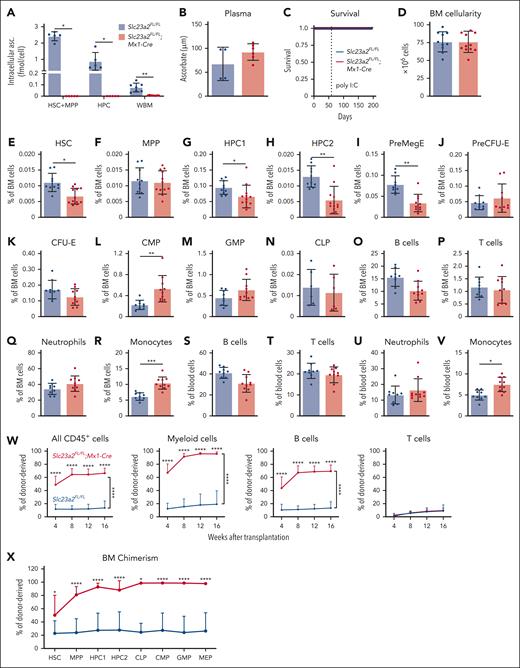
Because germ line Slc23a2 deficiency is embryonically lethal,35 we created a floxed allele of Slc23a2, thereby enabling conditional deletion from hematopoietic cells using Mx1-Cre (supplemental Figure 1A-C). Eight-week-old Slc23a2FL/FL;Mx1-Cre and Slc23a2FL/FL littermate control mice were injected with poly I:C to induce Cre expression and then analyzed 2 to 4 weeks later. Slc23a2 deletion did not alter the expression of other ascorbate transporters (supplemental Figure 1D).24,25,36-38 HSCs, MPPs, and HPC cells from Slc23a2FL/FL;Mx1-Cre mice contained no detectable intracellular ascorbate (Figure 1A). Ascorbate levels were 12-fold depleted in whole bone marrow cells from Slc23a2FL/FL;Mx1-Cre mice when compared with littermate controls (Figure 1A) but were normal in the blood plasma (Figure 1B), thus avoiding the development of scurvy (Figure 1C), which kills Gulo-deficient mice within 6 weeks in the absence of dietary supplementation with ascorbate.34Slc23a2 deficiency thus more severely depleted intracellular ascorbate from hematopoietic stem/progenitor cells when compared with Gulo-deficient mice fed a low ascorbate diet18 and avoided the potentially confounding effect of systemic ascorbate depletion.
Ascorbate deficiency increases the reconstituting potential of bone marrow cells upon transplantation into irradiated mice. (A-B) Ascorbate levels in HSCs, MPPs, HPCs, and whole bone marrow cells (A) and blood plasma (B) measured by mass spectrometry (a total of 5-7 mice per genotype in 2 independent experiments). (C) Kaplan-Meier survival curve of Slc23a2-deficient and littermate control mice (a total of 8 mice per genotype in 3 independent experiments). (D-V) Bone marrow cellularity from 2 tibias and 2 femurs (D), as well as the frequencies of HSCs (E), MPPs (F), restricted hematopoietic progenitors (G-N), and differentiated B cells (O,S), T cells (P,T), neutrophils (Q,U), and monocytes (R,V) in the bone marrow (D-R) and blood (S-V) (a total of 6-12 mice per genotype from 6 independent experiments). (W-X) Donor cell reconstitution (W) in the blood and bone marrow (X) after transplantation of 500 000 donor bone marrow cells from Slc23a2-deficient or littermate control mice, along with 2 000 000 competing wild-type cells into irradiated recipients (a total of 13-14 recipient mice per genotype transplanted with cells from 3 donors per genotype in 3 independent experiments). All data are presented as the mean ± standard deviation. Each dot in panels A-V represents a different mouse. Statistical significance was assessed using Student t tests (D), Mann-Whitney tests (B and time points in W), log-rank tests (C), Mann-Whitney tests with Holm-Sidak’s correction for multiple comparisons (A,E-V,X), or nparLD nonparametric mixed models with Holm-Sidak’s multiple comparisons corrections to test differences in overall reconstitution (W). All tests were 2-sided (∗P < .05; ∗∗P < .01; ∗∗∗P < .001; ∗∗∗∗P < .0001).
Ascorbate deficiency increases the reconstituting potential of bone marrow cells upon transplantation into irradiated mice. (A-B) Ascorbate levels in HSCs, MPPs, HPCs, and whole bone marrow cells (A) and blood plasma (B) measured by mass spectrometry (a total of 5-7 mice per genotype in 2 independent experiments). (C) Kaplan-Meier survival curve of Slc23a2-deficient and littermate control mice (a total of 8 mice per genotype in 3 independent experiments). (D-V) Bone marrow cellularity from 2 tibias and 2 femurs (D), as well as the frequencies of HSCs (E), MPPs (F), restricted hematopoietic progenitors (G-N), and differentiated B cells (O,S), T cells (P,T), neutrophils (Q,U), and monocytes (R,V) in the bone marrow (D-R) and blood (S-V) (a total of 6-12 mice per genotype from 6 independent experiments). (W-X) Donor cell reconstitution (W) in the blood and bone marrow (X) after transplantation of 500 000 donor bone marrow cells from Slc23a2-deficient or littermate control mice, along with 2 000 000 competing wild-type cells into irradiated recipients (a total of 13-14 recipient mice per genotype transplanted with cells from 3 donors per genotype in 3 independent experiments). All data are presented as the mean ± standard deviation. Each dot in panels A-V represents a different mouse. Statistical significance was assessed using Student t tests (D), Mann-Whitney tests (B and time points in W), log-rank tests (C), Mann-Whitney tests with Holm-Sidak’s correction for multiple comparisons (A,E-V,X), or nparLD nonparametric mixed models with Holm-Sidak’s multiple comparisons corrections to test differences in overall reconstitution (W). All tests were 2-sided (∗P < .05; ∗∗P < .01; ∗∗∗P < .001; ∗∗∗∗P < .0001).
The flow cytometry gates and cell surface markers used to identify each cell population characterized in this study are shown in supplemental Figure 1E-G and supplemental Table 1, respectively. Slc23a2 deficiency did not affect bone marrow cellularity (Figure 1D) or the frequencies of MPPs cells (Figure 1F-G), but it reduced the frequencies of HSCs (Figure 1E), HPC-1, and HPC2 cells (Figure 1G-H). Slc23a2 deficiency also reduced the frequency of megakaryocytic-erythroid progenitors (PreMegEs) (Figure 1I), but it did not change the frequency of erythroid progenitors (PreCFU-Es and CFU-Es) (Figure 1J-K). Slc23a2 deficiency increased the frequency of common myeloid progenitors (Figure 1L), but it did not significantly affect the frequencies of granulocyte-monocyte progenitors (GMPs) or common lymphoid progenitors (Figure 1M-N). Slc23a2 deficiency did not affect the frequencies of differentiated B cells, T cells, or neutrophils in the bone marrow or blood (Figure 1O-U), but it did increase the frequency of monocytes in the bone marrow (Figure 1R) and blood (Figure 1V). Overall, ascorbate deficiency in hematopoietic cells modestly depleted HSCs and increased monopoiesis in the bone marrow.
To test if Slc23a2 deficiency affects the reconstituting potential of bone marrow cells, we competitively transplanted whole bone marrow cells from Slc23a2FL/FL;Mx1-Cre and Slc23a2FL/FL control mice (2-4 weeks after poly I:C treatment) into irradiated mice. Consistent with our previous study,18Slc23a2-deficient bone marrow cells gave significantly higher levels of donor myeloid and B cell reconstitution than control bone marrow cells (Figure 1W). Slc23a2-deficient and control cells did not differ in terms of T-cell reconstitution in these experiments, however, this was not unexpected because ascorbate deficiency impairs T-cell differentiation in the thymus.23 At 16 weeks after transplantation, Slc23a2-deficient cells led to significantly higher donor contributions to HSCs and all hematopoietic progenitor cell populations we examined in the bone marrow when compared with control cells (Figure 1X). Therefore, ascorbate deficiency increased the reconstituting potential of bone marrow cells in irradiated mice despite a reduction in HSC frequency in the bone marrow.
Ascorbate deficiency increased HSC and MPP self-renewal potential
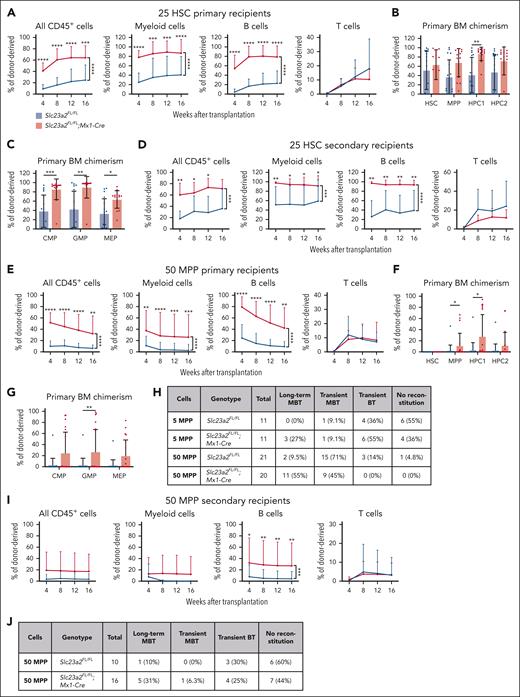
To better understand the increased reconstituting potential of ascorbate-depleted bone marrow cells, we competitively transplanted flow cytometrically isolated HSCs, MPPs, HPC1, or HPC2 cells from Slc23a2FL/FL;Mx1-Cre and Slc23a2FL/FL control mice (2-4 weeks after poly I:C treatment) into irradiated recipient mice. We first transplanted 25 donor HSCs per recipient from Slc23a2FL/FL;Mx1-Cre or littermate control mice into irradiated recipients. Nearly all recipient mice were long-term multilineage reconstituted by donor cells. When compared with control HSCs, Slc23a2-deficient HSCs gave significantly higher levels of donor myeloid and B cell reconstitution in the blood (Figure 2A), as well as donor HPC1 cell, common myeloid progenitors, GMP, and megakaryocyte-erythrocyte progenitor (MEP) reconstitution in the bone marrow (Figure 2B-C). We also serially transplanted whole bone marrow cells from these primary recipient mice into irradiated secondary recipients. The Slc23a2-deficient cells again gave significantly higher levels of donor myeloid and B cell reconstitution in the blood (Figure 2D).
Ascorbate deficiency increases the self-renewal potential of HSCs and MPPs. (A-C) Donor cell reconstitution in the blood (A) and bone marrow (B-C) of irradiated mice competitively transplanted with 25 HSCs from Slc23a2-deficient or littermate control mice (a total of 15-17 recipient mice per genotype transplanted with cells from 4 donors per genotype in 4 independent experiments). (D) Donor cell reconstitution in the blood of secondary recipients of 5 million bone marrow cells from the primary recipients in panel A (a total of 7-8 recipient mice per genotype transplanted with cells from 2 donor mice per genotype in 2 independent experiments). (E-G) Donor cell reconstitution in the blood (E) and bone marrow (F-G) of irradiated mice competitively transplanted with 50 MPPs from Slc23a2-deficient or littermate control mice (we did not detect any donor HSCs in these recipients) (a total of 20-21 recipient mice per genotype transplanted with cells from 5 donors per genotype in 5 independent experiments). (H) Summary of donor cell reconstitution profiles from primary recipients of MPPs. (I-J) Donor cell reconstitution in the blood of secondary recipients of bone marrow cells from the primary recipients in panel E (a total of 10-16 recipient mice per genotype transplanted with cells from 5 donor mice per genotype in 5 independent experiments). All data are presented as the mean ± standard deviation. Each dot in panels B-C,F-G represents a different mouse. Statistical significance was assessed using Mann-Whitney tests with Holm-Sidak’s corrections for multiple comparisons (B-C,F-G), Mann-Whitney tests to assess differences among genotypes at each timepoint, and nparLD models with Holm-Sidak’s multiple comparisons corrections to test differences among overall reconstitution (A,D-E,I). All statistical tests were 2-sided (∗P < .05; ∗∗P < .01; ∗∗∗P < .001; ∗∗∗∗P < .0001).
Ascorbate deficiency increases the self-renewal potential of HSCs and MPPs. (A-C) Donor cell reconstitution in the blood (A) and bone marrow (B-C) of irradiated mice competitively transplanted with 25 HSCs from Slc23a2-deficient or littermate control mice (a total of 15-17 recipient mice per genotype transplanted with cells from 4 donors per genotype in 4 independent experiments). (D) Donor cell reconstitution in the blood of secondary recipients of 5 million bone marrow cells from the primary recipients in panel A (a total of 7-8 recipient mice per genotype transplanted with cells from 2 donor mice per genotype in 2 independent experiments). (E-G) Donor cell reconstitution in the blood (E) and bone marrow (F-G) of irradiated mice competitively transplanted with 50 MPPs from Slc23a2-deficient or littermate control mice (we did not detect any donor HSCs in these recipients) (a total of 20-21 recipient mice per genotype transplanted with cells from 5 donors per genotype in 5 independent experiments). (H) Summary of donor cell reconstitution profiles from primary recipients of MPPs. (I-J) Donor cell reconstitution in the blood of secondary recipients of bone marrow cells from the primary recipients in panel E (a total of 10-16 recipient mice per genotype transplanted with cells from 5 donor mice per genotype in 5 independent experiments). All data are presented as the mean ± standard deviation. Each dot in panels B-C,F-G represents a different mouse. Statistical significance was assessed using Mann-Whitney tests with Holm-Sidak’s corrections for multiple comparisons (B-C,F-G), Mann-Whitney tests to assess differences among genotypes at each timepoint, and nparLD models with Holm-Sidak’s multiple comparisons corrections to test differences among overall reconstitution (A,D-E,I). All statistical tests were 2-sided (∗P < .05; ∗∗P < .01; ∗∗∗P < .001; ∗∗∗∗P < .0001).
We subsequently tested if Slc23a2 deficiency altered the reconstituting potential of MPPs by competitively transplanting 50 MPPs from Slc23a2FL/FL;Mx1-Cre or Slc23a2FL/FL control mice into irradiated recipients. When compared with control MPPs, Slc23a2-deficient MPPs gave significantly higher levels of donor myeloid and B cell reconstitution in the blood (Figure 2E), as well as donor MPP, HPC1 cell, and GMP reconstitution in the bone marrow (Figure 2F-G). Although most recipients of control MPPs were transiently reconstituted by donor cells (18 of 21 mice), most recipients of Slc23a2-deficient MPPs were long-term multilineage reconstituted by donor cells (11 of 20 mice) (Figure 2H).
We also competitively transplanted 5 MPPs from Slc23a2FL/FL;Mx1-Cre or Slc23a2FL/FL mice into irradiated recipients. Three of 11 primary recipients of Slc23a2-deficient cells were long-term multilineage reconstituted by donor cells, whereas none of the 11 primary recipients of control cells were long-term multilineage reconstituted by donor cells (Figure 2H). Limiting dilution analysis39 of the 5- and 50-cell transplant data indicated that Slc23a2 deficiency increased the frequency of long-term multilineage reconstituting cells in the MPP population by 10-fold (1 in 530 control MPPs and 1 in 52 Slc23a2-deficient MPPs; P = 1.1Â × 10−4). Ascorbate deficiency thus conferred long-term self-renewal potential to a subset of MPPs that normally only give transient multilineage reconstitution.9,40
To further test this, we serially transplanted bone marrow cells from recipients of 50 MPPs into irradiated secondary recipients. The Slc23a2-deficient cells again gave significantly higher levels of donor B-cell reconstitution in the blood of secondary recipients when compared with control cells (Figure 2I). Only 1 of 10 (10%) secondary recipients of control cells were long-term multilineage reconstituted, whereas 5 of 16 (31%) secondary recipients of Slc23a2-deficient cells were long-term multilineage reconstituted (Figure 2J).
We tested if Slc23a2 deficiency altered the reconstituting potential of HPC1 or HPC2 cells by competitively transplanting 500 HPC1 cells or 200 HPC2 cells from Slc23a2FL/FL;Mx1-Cre or Slc23a2FL/FL control mice into irradiated recipients. These cell populations normally only transiently reconstitute irradiated mice.40,41 Although Slc23a2-deficient HPC1 cells gave significantly higher levels of donor B-cell reconstitution than control HPC1 cells (supplemental Figure 2A), Slc23a2-deficient and control HPC1 and HPC2 cells gave only transient reconstitution (supplemental Figure 2B-D). Thus, ascorbate deficiency increased reconstitution by HPC1 and HPC2 cells but did not confer long-term reconstituting potential.
Ascorbate deficiency reduced cell division by HSCs and MPPs
To test if ascorbate deficiency altered the composition of the HSC and MPP pools, we assessed CD229 and CD244, markers that distinguish functionally distinct subsets of HSCs and MPPs.40Slc23a2 deficiency increased the frequency of HSC-1 (CD229−CD244−) cells while reducing the frequency of HSC-2 (CD229+CD244−) cells (Figure 3A-B). Because HSC-1 cells have increased self-renewal and reconstituting potential when compared with HSC-2 cells,40 this reflected a shift toward more primitive HSCs in ascorbate-depleted bone marrow. Slc23a2 deficiency also increased the frequency of MPP-1 (CD229−CD244−) cells while reducing the frequencies of MPP-2 (CD229+CD244−) and MPP-3 (CD229+ CD244+) cells (Figure 3A-B). Because MPP-1 cells have increased reconstituting potential when compared with MPP-2 and MPP-3 cells,40 this again reflected a shift toward more primitive MPPs in ascorbate-depleted bone marrow.
Ascorbate deficiency promotes HSC and MPP quiescence. (A) Representative flow cytometry plots showing staining for CD229 and CD244 in HSCs and MPPs from Slc23a2-deficient or littermate control mice. These markers distinguish functionally distinct subsets of HSCs and MPPs that differ in terms of primitiveness.40 (B) Frequencies of HSC-1, HSC-2, MPP-1, MPP-2, and MPP-3 subsets based on these markers (a total of 6 mice per genotype from 4 independent experiments). (C-D) Analysis of GO biological process terms that were enriched among genes downregulated (fold change, <0.5; false discovery rate, <0.01) in Slc23a2-deficient vs control HSCs (C) or MPPs (D) by RNA sequencing. (E-G) Single-cell RNA sequencing (scRNA-seq) UMAP (Uniform Manifold Approximation and Projection) plots of CD48−LSK cells (which contain HSCs and MPPs) from Slc23a2-deficient and control mice, including the percentages of cells that were positive for the cell cycle genes Mki67 (F) and Cyclin d1 (G). (H-I) Incorporation of a 3-day (H) or 2-hour (I) pulse of BrdU into HSCs, MPPs, HPC1 cells, HPC2 cells, and all c-kit+ progenitors (a total of 4-18 mice per genotype from 3 [I] and 7 [H] independent experiments). (J) Representative H2B-GFP fluorescence in HSCs and MPPs from Slc23a2FL/FL;Col1a1-H2B-GFP;Rosa26-M2-rtTA mice without doxycycline treatment (gray) or after 6 weeks of doxycycline treatment (red HSCs, green MPPs). (K) Representative H2B-GFP fluorescence in HSCs and MPPs from Slc23a2-deficient or littermate control mice after 12 weeks of chase without doxycycline. (L) The percentages of H2B-GFPhigh, H2B-GFPlow, or H2B-GFPneg HSCs and MPPs after 12 weeks of chase without doxycycline (a total of 8 mice per genotype analyzed in 4 independent experiments). All data are presented as the mean ± standard deviation. Each dot represents a different mouse in panels B,H-I,L. Statistical significance was assessed using Student t tests with Holm-Sidak’s correction for multiple comparisons (B,H-I) or Mann-Whitney tests with Holm-Sidak’s corrections for multiple comparisons (L). All statistical tests were 2-sided (∗P < .05; ∗∗P < .01; ∗∗∗P < .001).
Ascorbate deficiency promotes HSC and MPP quiescence. (A) Representative flow cytometry plots showing staining for CD229 and CD244 in HSCs and MPPs from Slc23a2-deficient or littermate control mice. These markers distinguish functionally distinct subsets of HSCs and MPPs that differ in terms of primitiveness.40 (B) Frequencies of HSC-1, HSC-2, MPP-1, MPP-2, and MPP-3 subsets based on these markers (a total of 6 mice per genotype from 4 independent experiments). (C-D) Analysis of GO biological process terms that were enriched among genes downregulated (fold change, <0.5; false discovery rate, <0.01) in Slc23a2-deficient vs control HSCs (C) or MPPs (D) by RNA sequencing. (E-G) Single-cell RNA sequencing (scRNA-seq) UMAP (Uniform Manifold Approximation and Projection) plots of CD48−LSK cells (which contain HSCs and MPPs) from Slc23a2-deficient and control mice, including the percentages of cells that were positive for the cell cycle genes Mki67 (F) and Cyclin d1 (G). (H-I) Incorporation of a 3-day (H) or 2-hour (I) pulse of BrdU into HSCs, MPPs, HPC1 cells, HPC2 cells, and all c-kit+ progenitors (a total of 4-18 mice per genotype from 3 [I] and 7 [H] independent experiments). (J) Representative H2B-GFP fluorescence in HSCs and MPPs from Slc23a2FL/FL;Col1a1-H2B-GFP;Rosa26-M2-rtTA mice without doxycycline treatment (gray) or after 6 weeks of doxycycline treatment (red HSCs, green MPPs). (K) Representative H2B-GFP fluorescence in HSCs and MPPs from Slc23a2-deficient or littermate control mice after 12 weeks of chase without doxycycline. (L) The percentages of H2B-GFPhigh, H2B-GFPlow, or H2B-GFPneg HSCs and MPPs after 12 weeks of chase without doxycycline (a total of 8 mice per genotype analyzed in 4 independent experiments). All data are presented as the mean ± standard deviation. Each dot represents a different mouse in panels B,H-I,L. Statistical significance was assessed using Student t tests with Holm-Sidak’s correction for multiple comparisons (B,H-I) or Mann-Whitney tests with Holm-Sidak’s corrections for multiple comparisons (L). All statistical tests were 2-sided (∗P < .05; ∗∗P < .01; ∗∗∗P < .001).
To further explore this, we sequenced RNA from Slc23a2-deficient and control HSCs and MPPs. We identified that the expression of 81 and 65 genes was significantly reduced and that the expression of 9 and 19 genes was significantly increased in Slc23a2-deficient vs control HSCs and MPPs, respectively (fold change, >twofold; false discovery rate, <0.01; supplemental Table 2). However, Slc23a2 deficiency did not significantly change the expression of markers that distinguish HSCs from MPPs in either HSCs or MPPs, suggesting that ascorbate deficiency did not cause phenotypic changes in HSCs that made them resemble MPPs (supplemental Figure 3A-R).41,42 Gene ontology (GO) term analysis revealed that all 10 of the most significantly downregulated GO terms in Slc23a2-deficient vs control HSCs and MPPs were related to cell division (Figure 3C-D). No GO terms were significantly enriched among the genes that were upregulated in Slc23a2-deficient HSCs when compared with control HSCs. This suggested that the main difference in gene expression among Slc23a2-deficient vs control HSCs and MPPs reflected reduced cell division among the Slc23a2-deficient cells.
We also performed single-cell RNA sequencing in CD48−Lineage−Sca1+c-kit+ cells (which comprise HSCs and MPPs) from Slc23a2FL/FL;Mx1-Cre and Slc23a2FL/FL control mice. Slc23a2-deficient cells did not cluster differently from control cells (Figure 3E). We identified HSCs and MPPs in the single-cell RNA sequencing data using published HSC and MPP gene signatures.42 Again, Slc23a2-deficient HSCs and MPPs did not cluster differently from control HSCs and MPPs (Figure 3E). Only 3 genes were significantly differentially expressed among Slc23a2-deficient and control CD48−Lineage−Sca1+c-kit+ cells, namely expression of the cell cycle genes Mki67 and Hist1h1b was reduced , whereas the expression of Fosb was increased. Paralogs of Fosb promote HSC quiescence.43,44 Finally, Slc23a2 deficiency reduced the percentage of HSCs and MPPs that expressed the G1 marker Ccnd1 (which encodes cyclin D1) and the proliferation marker Mki67 (which encodes Ki67; Figure 3F-G). These data further indicated that Slc23a2 deficiency reduced HSC and MPP cell division.
To test this directly, we administered a 3-day pulse of 5-bromo-3-deoxyuridine (BrdU) to Slc23a2FL/FL;Mx1-Cre and Slc23a2FL/FL control mice and then assessed BrdU incorporation into HSCs and MPPs by flow cytometry. Significantly lower percentages of Slc23a2-deficient HSCs and MPPs incorporated BrdU when compared with control HSCs and MPPs (Figure 3H). However, we observed no significant difference in BrdU incorporation into Slc23a2-deficient HPC1 cells, HPC2 cells, or c-kit+ cells when compared with control cells (Figure 3I). Consistent with a previous study,18 less severe ascorbate depletion in Gulo-deficient mice fed a low ascorbate diet did not significantly change the composition of the HSC and MPP pools or their rate of BrdU incorporation (supplemental Figure 3U-V). Ascorbate deficiency thus selectively reduced the rates of HSC and MPP cell division.
Ascorbate exists in a series of redox states that can act either as an antioxidant or a pro-oxidant.45,46 Consistent with a previous study,18 we did not detect significant differences in the levels of reactive oxygen species between Slc23a2-deficient and control HSCs and MPPs (supplemental Figure 4A-C).
We also deleted Slc23a2 from HSCs using Scl-CreER, which recombines in HSCs, MPPs, and other early hematopoietic progenitors.32 Five weeks after tamoxifen administration, HSCs and MPPs from Slc23a2FL/FL;Scl-CreER mice exhibited significantly reduced BrdU incorporation when compared with HSCs and MPPs from Slc23a2FL/FL control mice (supplemental Figure 4D-E).
To test whether ascorbate deficiency in HSCs and MPPs acts cell-autonomously to increase the reconstituting potential, we competitively transplanted donor bone marrow cells from Slc23a2FL/FL;Mx1-Cre and Slc23a2FL/FL mice into irradiated recipients. Five weeks later, we treated the mice with poly I:C to delete Slc23a2. The Slc23a2-deficient cells gave significantly higher levels of donor myeloid and B-cell reconstitution than control donor cells (supplemental Figure 4F). Ascorbate deficiency thus acted cell-autonomously to increase the reconstituting potential of bone marrow cells.
Ascorbate deficiency conferred long-term self-renewal potential to quiescent MPPs
To more deeply assess the effect of ascorbate deficiency on HSC and MPP cell division, we mated Slc23a2FL/FL;Mx1-Cre mice with Col1a1-H2B-GFP;Rosa26-M2-rtTA mice15 and then administered 6 weeks of doxycycline to label cells with H2B-GFP. We then withdrew the doxycycline so that the H2B-GFP levels would be diluted with each round of cell division over a subsequent 12-week chase (Figure 3J-K).14,15 After 12 weeks of chase, we quantified the percentages of HSCs and MPPs that had undergone only a few divisions (H2B-GFPhigh), an intermediate number of divisions (H2B-GFPlow), or many divisions (H2B-GFPneg; Figure 3G-H). Slc23a2 deficiency substantially increased H2B-GFP retention in both HSCs and MPPs. This was reflected by significantly higher percentages of H2B-GFPhigh HSCs and MPPs and significantly lower percentages of H2B-GFPlow and H2B-GFPneg HSCs and MPPs in Slc23a2-deficient mice than in control mice (Figure 3L). HSCs and MPPs thus undergo prolonged quiescence when deficient in ascorbate.
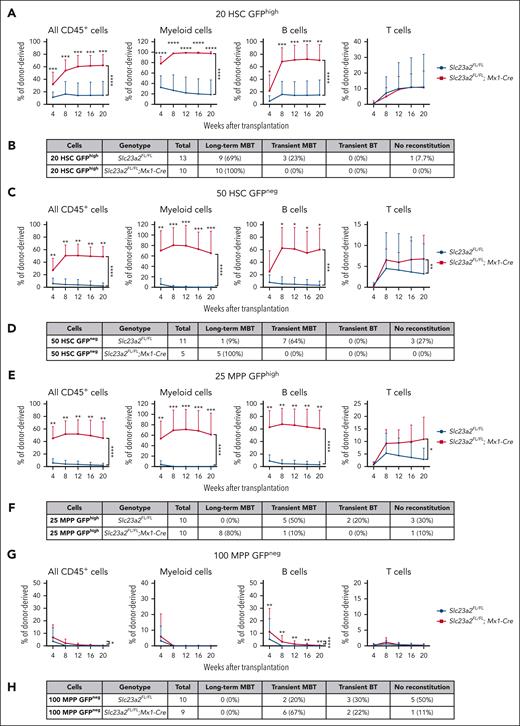
To test if reduced cell division was associated with increased reconstituting potential in HSCs, we competitively transplanted 20 H2B-GFPhigh HSCs or 50 H2B-GFPneg HSCs from Slc23a2-deficient and control mice after 12 weeks of chase. As expected,14,15 among control cells, the H2B-GFPhigh HSCs gave significantly higher levels of donor myeloid cell reconstitution and a higher percentage of recipients that were long-term multilineage reconstituted than H2B-GFPneg HSCs (Figure 4A-D). Slc23a2-deficient H2B-GFPhigh HSCs and Slc23a2-deficient H2B-GFPneg HSCs each gave significantly higher levels of donor myeloid and B-cell reconstitution and a higher percentage of recipients that were long-term multilineage reconstituted than control H2B-GFPhi HSCs and control H2B-GFPneg HSCs, respectively (Figure 4A-D). Because H2B-GFPneg HSCs were very rare in Slc23a2-deficient mice (Figure 3I), only a few of these cells could be isolated for transplantation. Nonetheless, all recipients that were transplanted with Slc23a2-deficient H2B-GFPneg HSCs were long-term multilineage reconstituted by donor cells, whereas only 1 of 9 recipients of control H2B-GFPneg HSCs were long-term multilineage reconstituted by donor cells (Figure 4D). Ascorbate deficiency thus increased the reconstituting potential in both frequently dividing and rarely dividing HSCs.
Ascorbate deficiency confers long-term reconstituting potential to quiescent MPPs. (A) Donor cell reconstitution in the blood of irradiated mice competitively transplanted with 20 H2B-GFPhigh HSCs isolated from Slc23a2-deficient or littermate control mice (a total of 10-13 recipient mice per genotype transplanted with cells from 3 donors per genotype in 3 independent experiments). (B) Summary of the donor cell reconstitution profiles in panel A. (C) Donor cell reconstitution in mice competitively transplanted with 50 H2B-GFPneg HSCs isolated from Slc23a2-deficient or littermate control mice (a total of 2-10 recipient mice per genotype transplanted with cells from 2 donors per genotype in 2 independent experiments). (D) Summary of the donor cell reconstitution profiles in panel C. (E) Donor cell reconstitution in mice competitively transplanted with 25 H2B-GFPhigh MPPs isolated from Slc23a2-deficient or littermate control mice (a total of 10 recipient mice per genotype transplanted with cells from 2 donors per genotype in 2 independent experiments). (F) Summary of the donor cell reconstitution profiles in panel E. (G) Donor cell reconstitution in mice competitively transplanted with 100 H2B-GFPneg MPPs isolated from Slc23a2-deficient or littermate control mice (a total of 6-8 recipient mice per genotype transplanted with cells from 2 donors per genotype in 2 independent experiments). (H) Summary of the donor cell reconstitution profiles in panel G. All data are presented as the mean ± standard deviation. Statistical significance was assessed using Mann-Whitney tests to assess differences among genotypes at each timepoint and nparLD models with Holm-Sidak’s multiple comparisons corrections to test differences among genotypes in the overall reconstitution (A,C,E,G). All statistical tests were 2-sided (∗P < .05; ∗∗P < .01; ∗∗∗P < .001; ∗∗∗∗P < .0001).
Ascorbate deficiency confers long-term reconstituting potential to quiescent MPPs. (A) Donor cell reconstitution in the blood of irradiated mice competitively transplanted with 20 H2B-GFPhigh HSCs isolated from Slc23a2-deficient or littermate control mice (a total of 10-13 recipient mice per genotype transplanted with cells from 3 donors per genotype in 3 independent experiments). (B) Summary of the donor cell reconstitution profiles in panel A. (C) Donor cell reconstitution in mice competitively transplanted with 50 H2B-GFPneg HSCs isolated from Slc23a2-deficient or littermate control mice (a total of 2-10 recipient mice per genotype transplanted with cells from 2 donors per genotype in 2 independent experiments). (D) Summary of the donor cell reconstitution profiles in panel C. (E) Donor cell reconstitution in mice competitively transplanted with 25 H2B-GFPhigh MPPs isolated from Slc23a2-deficient or littermate control mice (a total of 10 recipient mice per genotype transplanted with cells from 2 donors per genotype in 2 independent experiments). (F) Summary of the donor cell reconstitution profiles in panel E. (G) Donor cell reconstitution in mice competitively transplanted with 100 H2B-GFPneg MPPs isolated from Slc23a2-deficient or littermate control mice (a total of 6-8 recipient mice per genotype transplanted with cells from 2 donors per genotype in 2 independent experiments). (H) Summary of the donor cell reconstitution profiles in panel G. All data are presented as the mean ± standard deviation. Statistical significance was assessed using Mann-Whitney tests to assess differences among genotypes at each timepoint and nparLD models with Holm-Sidak’s multiple comparisons corrections to test differences among genotypes in the overall reconstitution (A,C,E,G). All statistical tests were 2-sided (∗P < .05; ∗∗P < .01; ∗∗∗P < .001; ∗∗∗∗P < .0001).
We also competitively transplanted 25 H2B-GFPhigh MPPs or 100 H2B-GFPneg MPPs from Slc23a2-deficient and control mice into irradiated recipients. Slc23a2-deficient H2B-GFPhigh MPPs gave significantly higher levels of donor myeloid, B-, and T-cell reconstitution than control H2B-GFPhigh MPPs (Figure 4E); 8 of 10 recipients of Slc23a2-deficient H2B-GFPhigh MPPs were long-term multilineage reconstituted by donor cells, whereas none of the 10 recipients of control H2B-GFPhigh MPPs were long-term multilineage reconstituted (Figure 4F). Slc23a2-deficient H2B-GFPneg MPPs gave significantly higher levels of donor B-cell reconstitution than control H2B-GFPneg MPPs (Figure 4G); however, none of the recipients of Slc23a2-deficient or control H2B-GFPneg MPPs were long-term multilineage reconstituted (Figure 4H). Ascorbate deficiency thus conferred long-term reconstituting potential to quiescent but not frequently dividing MPPs.
Ascorbate deficiency does not promote MPP self-renewal via reduced Tet2 function
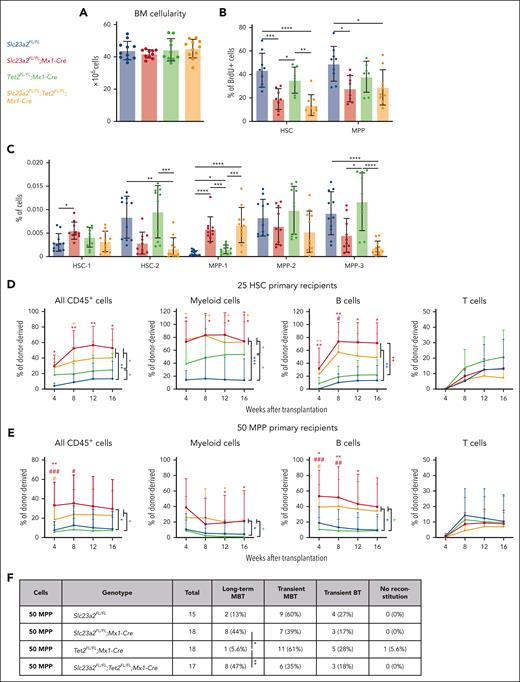
Ascorbate depletion in the context of healthy Gulo-deficient mice increases HSC reconstituting potential partly by reducing the function of Tet dioxygenases, particularly Tet2.18,47 We thus tested whether the more severe ascorbate deficiency in Slc23a2-deficient mice altered HSC and MPP function by reducing Tet2 function. To do this, we generated Slc23a2FL/FL;Tet2FL/FL;Mx1-Cre double mutant mice, Slc23a2FL/FL;Mx1-Cre and Tet2FL/FL;Mx1-Cre single mutant mice, and Slc23a2FL/FL controls. Tet2, Slc23a2, and Slc23a2/Tet2 mutant mice did not significantly differ from control mice in terms of bone marrow cellularity (Figure 5A). Relative to control mice, Slc23a2 deficiency significantly reduced BrdU incorporation in HSCs and MPPs but Tet2 deficiency did not and Slc23a2/Tet2 deficient HSCs and MPPs did not significantly differ from Slc23a2 deficient HSCs and MPPs (Figure 5B). We also examined the composition of the HSC and MPP pools in these mice. Relative to control mice, Slc23a2 deficiency significantly increased the frequencies of HSC-1 and MPP-1 cells but Tet2 deficiency did not, and Slc23a2/Tet2 double deficient mice did not significantly differ from Slc23a2 deficient mice (Figure 5C). Thus, Tet2 deficiency did not phenocopy the effect of Slc23a2 deficiency on HSC and MPP cell division or pool composition, suggesting that the effect of ascorbate deficiency on HSCs and MPPs is not mediated by a reduction in Tet2 function.
Tet2 does not seem to mediate the effects of ascorbate deficiency on the reconstituting potential of MPPs. Each panel in this figure compares cells from control mice, Slc23a2FL/FL;Mx1-Cre mice, Tet2FL/FL;Mx1-Cre mice, and Slc23a2FL/FL;Tet2FL/FL;Mx1-Cre mice. (A) Bone marrow cellularity in 1 tibia and 1 femur (a total of 10-11 mice per genotype in 6 independent experiments). (B) Incorporation of a 3-day BrdU pulse in HSCs and MPPs (a total of 7-9 mice per genotype in 6 independent experiments). (C) Frequencies of HSC-1, HSC-2, MPP-1, MPP-2, and MPP-3 cells in the bone marrow (a total of 10-11 mice per genotype in 6 independent experiments). (D) Donor cell reconstitution in the blood of irradiated mice competitively transplanted with 25 HSCs. For individual timepoints, ∗ indicates comparison with wild type controls and # indicates comparison with Tet2-deficient cells (a total of 6-9 recipient mice per genotype transplanted with cells from 2 donors per genotype in 2 independent experiments). (E) Donor cell reconstitution in the blood of irradiated mice competitively transplanted with 50 MPPs (a total of 15-18 recipient mice per genotype transplanted with cells from 5 donors per genotype in 5 independent experiments). (F) Summary of the donor cell reconstitution profiles in panel E. All data are presented as the mean ± standard deviation. Each dot represents a different mouse in panels A-C. Statistical significance was assessed using 1-way analysis of variance tests with Tukey's correction for multiple comparisons (A-B), Kruskal-Wallis tests with Dunn's correction for multiple comparisons (C), Kruskal-Wallis tests with Dunn's correction for multiple comparisons to assess differences among genotypes within each timepoint, and nparLD models with the false discovery rate method for multiple comparison corrections to test differences among genotypes in overall reconstitution (D-E). A Fisher exact test was used to test differences among genotypes in the number of long-term multilineage reconstituted mice (F). All statistical tests were 2-sided (∗P < .05; ∗∗P < .01; ∗∗∗P < .001; ∗∗∗∗P < .0001).
Tet2 does not seem to mediate the effects of ascorbate deficiency on the reconstituting potential of MPPs. Each panel in this figure compares cells from control mice, Slc23a2FL/FL;Mx1-Cre mice, Tet2FL/FL;Mx1-Cre mice, and Slc23a2FL/FL;Tet2FL/FL;Mx1-Cre mice. (A) Bone marrow cellularity in 1 tibia and 1 femur (a total of 10-11 mice per genotype in 6 independent experiments). (B) Incorporation of a 3-day BrdU pulse in HSCs and MPPs (a total of 7-9 mice per genotype in 6 independent experiments). (C) Frequencies of HSC-1, HSC-2, MPP-1, MPP-2, and MPP-3 cells in the bone marrow (a total of 10-11 mice per genotype in 6 independent experiments). (D) Donor cell reconstitution in the blood of irradiated mice competitively transplanted with 25 HSCs. For individual timepoints, ∗ indicates comparison with wild type controls and # indicates comparison with Tet2-deficient cells (a total of 6-9 recipient mice per genotype transplanted with cells from 2 donors per genotype in 2 independent experiments). (E) Donor cell reconstitution in the blood of irradiated mice competitively transplanted with 50 MPPs (a total of 15-18 recipient mice per genotype transplanted with cells from 5 donors per genotype in 5 independent experiments). (F) Summary of the donor cell reconstitution profiles in panel E. All data are presented as the mean ± standard deviation. Each dot represents a different mouse in panels A-C. Statistical significance was assessed using 1-way analysis of variance tests with Tukey's correction for multiple comparisons (A-B), Kruskal-Wallis tests with Dunn's correction for multiple comparisons (C), Kruskal-Wallis tests with Dunn's correction for multiple comparisons to assess differences among genotypes within each timepoint, and nparLD models with the false discovery rate method for multiple comparison corrections to test differences among genotypes in overall reconstitution (D-E). A Fisher exact test was used to test differences among genotypes in the number of long-term multilineage reconstituted mice (F). All statistical tests were 2-sided (∗P < .05; ∗∗P < .01; ∗∗∗P < .001; ∗∗∗∗P < .0001).
Next, we competitively transplanted 25 HSCs into irradiated mice and assessed donor cell reconstitution. Relative to control HSCs, Slc23a2-deficient HSCs gave significantly higher levels of donor myeloid and B-lineage reconstitution (Figure 5D). Relative to control HSCs, Tet2-deficient HSCs gave significantly higher levels of donor myeloid but not B-cell reconstitution (Figure 5D). Slc23a2/Tet2–double deficient HSCs gave similar levels of donor cell reconstitution as Slc23a2-deficient HSCs (Figure 5D). This is consistent with the conclusion that ascorbate deficiency increases the myeloid but not B-lineage reconstituting potential of HSCs by reducing Tet2 function. Together with previous studies,18,47 our data indicate that ascorbate regulates HSC reconstituting potential through Tet2-dependent and independent mechanisms.
We also competitively transplanted 50 MPPs into irradiated recipient mice and assessed donor cell reconstitution. Relative to control MPPs, Slc23a2-deficient MPPs and Slc23a2/Tet2–double deficient MPPs gave significantly higher levels of donor myeloid and B-lineage reconstitution (Figure 5E); however, Tet2-deficient MPPs gave similar levels of reconstitution as control MPPs (Figure 5E). Only 2 of 15 recipients of control MPPs and 1 of 18 recipients of Tet2 deficient MPPs were long-term multilineage reconstituted by donor cells (Figure 5E-F). In contrast, 8 of 18 recipients of Slc23a2-deficient MPPs and 8 of 17 recipients of Slc23a2/Tet2-deficient MPPs were long-term multilineage reconstituted by donor cells. Tet2 deficiency, therefore, did not phenocopy the effect of ascorbate deficiency on MPP reconstituting potential. Ascorbate deficiency seems to increase the reconstituting potential and self-renewal potential of MPPs through mechanisms independent of Tet2 function.
Although mouse HSCs and MPPs express Slc23a2 but not Slc23a1 (supplemental Figure 1D) and depend exclusively on Slc23a2 for ascorbate uptake (Figure 1A), human hematopoietic stem and progenitor cells express both SLC23A1 and SLC23A2 (supplemental Figure 4G).48,49 Consequently, we would not expect human stem/progenitor cells to depend exclusively on SLC23A2 for ascorbate uptake. Nonetheless, ascorbate supplementation to superphysiological levels increases 5-hydroxy-methyl-cytosine levels and reduces the expansion of human TET2 mutant hematopoietic stem/progenitor cells xenografted in mice,50 consistent with similar effects observe in mouse cells.47 Moreover, patients with hematological malignancies have low plasma ascorbate levels51 and oral ascorbate supplementation may increase the survival of patients with low-risk myeloid malignancies,52 similar to what we observed in mouse leukemia.18
Discussion
We reported previously that ascorbate limits HSC function during reconstitution of irradiated mice18; however, the effect of ascorbate on MPP function had not been tested. In this study, we characterized the effect of ascorbate deficiency on hematopoietic cells by deleting the Slc23a2 ascorbate transporter (Figure 1A). We were surprised that this not only increased HSC function but also conferred long-term reconstituting potential to MPPs (Figure 2E-J). Deletion of Slc23a2 from hematopoietic cells increased quiescence (Figure 3H-L), reconstituting potential (Figure 2A-C,E-G), and self-renewal potential (Figure 2D,H-J) in both HSCs and MPPs. Other essential dietary nutrients also regulate HSC function, including vitamin A20,53 and valine.54 However, in contrast with ascorbate, dietary depletion of vitamin A or valine undermines HSC function.
Ascorbate deficiency increased the reconstituting potential of both dividing and nondividing HSCs (Figure 4A-D), but it only significantly increased the reconstituting potential of the nondividing (or slowly dividing) fraction of MPPs (Figure 4E-H). When combined with the recent observation of higher ascorbate levels in dividing as opposed to quiescent HSCs,21 these observations suggest that ascorbate promotes cell division in HSCs and MPPs, at least under steady-state conditions. Nonetheless, ascorbate-deficient HSCs and MPPs were capable of cell division because they gave higher levels of reconstitution than control cells in irradiated mice.
Much of the effect of ascorbate on HSC function is mediated by Tet2.18 Superphysiological ascorbate levels attenuate the effects of Tet2 deficiency in mouse and human HSCs,47,50 partly by promoting Tet3 function.47 Furthermore, ascorbate promotes plasma cell differentiation by increasing Tet2 and Tet3 function.28 However, the effects of ascorbate deficiency on MPP reconstituting potential do not seem to be mediated by Tet2 because Tet2 deficiency did not phenocopy the effects of ascorbate deficiency on MPPs (Figure 5B-C,E-F).55 The expression of many cell cycle–related genes were reduced in ascorbate-deficient vs control MPPs (Figure 3C-D), consistent with the increased quiescence of Slc23a2-deficient MPPs. However, the mechanism by which ascorbate promotes cell division and limits self-renewal potential in MPPs remains unclear.
Acknowledgments
The authors thank Jian Xu and Michalis Agathocleous for helpful discussions and Michael Ortiz and the Moody Foundation Flow Cytometry Facility, Tripti Sharma and the Mouse Genome Engineering Facility, and the Metabolomics Facility in the Children’s Medical Center Research Institute at UT Southwestern. The authors also thank Megan Mulkey for mouse colony management and the BioHPC High-Performance Facility Cloud at UT Southwestern Medical Center for providing computational resources.
S.J.M. is a Howard Hughes Medical Institute Investigator, the Mary McDermott Cook Chair in Pediatric Genetics, the Kathryn and Gene Bishop Distinguished Chair in Pediatric Research, the director of the Hamon Laboratory for Stem Cells and Cancer. S.J.M. is a Cancer Prevention and Research Institute of Texas Scholar. This work was supported by the National Institutes of Health (NIH), National Institute of Diabetes and Digestive and Kidney Diseases (grant DK118745) and the Kleberg Foundation. S.C. was supported by an EMBO Long-Term Fellowship (grant ALTF 722-2015), E.C.J. was supported by a postdoctoral fellowship from the Damon Runyon Cancer Research Foundation (grant 2278-16), and A.W.D. was supported by a Ruth L. Kirschstein National Research Service Award Postdoctoral fellowship from the NIH, National Heart, Lung, and Blood Institute (grant F32HL135975).
Authorship
Contribution: S.C. and S.J.M. conceived the project and designed and interpreted experiments; S.C. performed most of the experiments with technical assistance from D.L.C., A.W.D., E.C.J., B.R.O., A.P., S.M., T.P.M., and A.R.R.; B.C. and Z.Z. performed the RNA-seq, single-cell RNA sequencing, and statistical analyses; S.B. and S.Z.X. provided gene expression data from human cells; and S.C. and S.J.M. wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
The current affiliation for S.C. is Centre for Regenerative Medicine, Institute for Regeneration and Repair, The University of Edinburgh, Edinburgh, United Kingdom.
Correspondence: Sean J. Morrison, Children’s Research Institute and the Department of Pediatrics, University of Texas Southwestern Medical Center, Dallas, TX 75390-8502; email: sean.morrison@utsouthwestern.edu.
References
Author notes
The authors will make all materials available upon publication. The RNA-seq data can be accessed at the National Center for Biotechnology Information Sequence Read Archive (Bioproject ID: PRJNA1091339).
The online version of this article contains a data supplement.
There is a Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.

![Ascorbate deficiency promotes HSC and MPP quiescence. (A) Representative flow cytometry plots showing staining for CD229 and CD244 in HSCs and MPPs from Slc23a2-deficient or littermate control mice. These markers distinguish functionally distinct subsets of HSCs and MPPs that differ in terms of primitiveness.40 (B) Frequencies of HSC-1, HSC-2, MPP-1, MPP-2, and MPP-3 subsets based on these markers (a total of 6 mice per genotype from 4 independent experiments). (C-D) Analysis of GO biological process terms that were enriched among genes downregulated (fold change, <0.5; false discovery rate, <0.01) in Slc23a2-deficient vs control HSCs (C) or MPPs (D) by RNA sequencing. (E-G) Single-cell RNA sequencing (scRNA-seq) UMAP (Uniform Manifold Approximation and Projection) plots of CD48−LSK cells (which contain HSCs and MPPs) from Slc23a2-deficient and control mice, including the percentages of cells that were positive for the cell cycle genes Mki67 (F) and Cyclin d1 (G). (H-I) Incorporation of a 3-day (H) or 2-hour (I) pulse of BrdU into HSCs, MPPs, HPC1 cells, HPC2 cells, and all c-kit+ progenitors (a total of 4-18 mice per genotype from 3 [I] and 7 [H] independent experiments). (J) Representative H2B-GFP fluorescence in HSCs and MPPs from Slc23a2FL/FL;Col1a1-H2B-GFP;Rosa26-M2-rtTA mice without doxycycline treatment (gray) or after 6 weeks of doxycycline treatment (red HSCs, green MPPs). (K) Representative H2B-GFP fluorescence in HSCs and MPPs from Slc23a2-deficient or littermate control mice after 12 weeks of chase without doxycycline. (L) The percentages of H2B-GFPhigh, H2B-GFPlow, or H2B-GFPneg HSCs and MPPs after 12 weeks of chase without doxycycline (a total of 8 mice per genotype analyzed in 4 independent experiments). All data are presented as the mean ± standard deviation. Each dot represents a different mouse in panels B,H-I,L. Statistical significance was assessed using Student t tests with Holm-Sidak’s correction for multiple comparisons (B,H-I) or Mann-Whitney tests with Holm-Sidak’s corrections for multiple comparisons (L). All statistical tests were 2-sided (∗P < .05; ∗∗P < .01; ∗∗∗P < .001).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/145/1/10.1182_blood.2024024769/5/m_blood_bld-2024-024769-gr3.jpeg?Expires=1769114772&Signature=EWNywtaqKRG3oQxgnh~L8S4M8eB8OVeMxNYcjvZIbyGBCyS7qaly~b4OevRqlOjJzomTkUps1amt07pK~GPXKmq7Jq0MB3DaNbl3JbFoqM9EHODk1iQXRHDm03dHn8epGgmiLSoKuybX5~aVQn8f~2H9O6vkxY-gm15f36wHl00ZexJNAT64wcEVk1Oha12hFAr3S5gihiBUXsE~t9Z2V2ihWunNfzh3ZeqiI7R-kuiiguTaPG5-BS3-JJ7wM0LiSm3hOjyBhDTM8qo2ulf3c80MajsyXsW45t1VzmB1Xxwqe8Tl4kCbSz-AgpktN7gyQJKa-sttvDu8JNjupOFf1w__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal