Key Points
Iptacopan monotherapy is cost saving for anti-C5–treated patients with suboptimal response vs continuation of C5 inhibitors.
Improved quality of life is linked to greater transfusion independence and the overall convenience of oral therapy.
Visual Abstract
Iptacopan, a novel oral factor B inhibitor, recently obtained US Food and Drug Administration approval for treating paroxysmal nocturnal hemoglobinuria, a rare blood disorder characterized by persistent complement-mediated hemolytic anemia. The standard-of-care (SOC) has traditionally relied on complement C5 inhibitors eculizumab and ravulizumab, which are limited by persistent anemia from extravascular hemolysis and requirement for intravenous infusion. Recent publication of phase 3 studies in this arena reinforces iptacopan as an effective anticomplement monotherapy compared with SOC. Given ongoing price negotiations and limited literature showing its cost-ineffectiveness in the anti-C5–treated population, we conducted a comprehensive cost-effectiveness analysis of iptacopan monotherapy in anti-C5–treated patients from the societal perspective, as compared with C5 inhibition. The primary outcomes were the incremental net monetary benefit across a lifetime horizon and the cost-effective maximum monthly threshold price of iptacopan monotherapy compared with the SOC. The secondary outcome was time saved for patients and nurses with the use of oral iptacopan therapy. Iptacopan monotherapy and SOC accrued 12.6 and 10.8 quality-adjusted life-years at costs of $9.52 million and $13.5 million, respectively. Iptacopan monotherapy remained cost saving across extensive sensitivity and all scenario analyses, including alternative parameterization for anemia resolution and aggregated individual-level utilities and transition probability matrix. Across all probabilistic sensitivity analyses, iptacopan monotherapy was favored over SOC in 100% of 10 000 Monte Carlo iterations. Cost-saving thresholds for iptacopan vs anti-C5 are ∼1.1, 1.4, and 1.4 in Brazil, Japan, and the United States, respectively. Iptacopan monotherapy can improve quality-adjusted life expectancy for patients while saving health care costs across jurisdictions.
Introduction
Paroxysmal nocturnal hemoglobinuria (PNH) is a rare, acquired blood disorder characterized by life-threatening complications that include hemolytic anemia, bone marrow failure, and thrombosis.1 Mechanistically, these complications are driven by the loss of anticomplement factors on hematopoietic cells, leading to chronic complement cascade activation. For patients living with PNH, the standard of care since 2007 has anchored on complement C5 inhibition with eculizumab, broadening to include ravulizumab in 2018, with supplemental transfusions as needed.2-4 Of note, anti-C5 therapeutic effectiveness can be limited by the persistence of C3-mediated extravascular hemolysis, which contributes to residual anemia in up to 72% of patients with PNH. Moreover, given their intravenous administration, C5 inhibitors also require significant patient and clinic time for regular infusions.5-8
In the past several years, proximal targets for complement cascade inhibition have emerged as additional treatment options. For example, pegcetacoplan, a subcutaneously administered C3 inhibitor approved in 2021, demonstrated improved clinical efficacy over eculizumab, ultimately improving anemia-related symptoms.9,10 However, the adoption of pegcetacoplan has been limited by its infusion system and concerns for intravascular hemolysis from incomplete complement inhibition.11,12 More recently, iptacopan, an oral factor B inhibitor, was shown to provide durable responses for patients living with PNH. In the randomized phase 3 APPLY-PNH trial (ClinicalTrials.gov identifier: NCT04558918), patients with PNH on anti-C5 therapy were randomized to iptacopan or continuation of C5 inhibition. Patients in the iptacopan group experienced higher hemoglobin levels, higher rates of transfusion independence, and reductions in patient-reported fatigue.13 These results, in combination with data from the single-arm phase 3 APPOINT-PNH trial (ClinicalTrials.gov identifier: NCT04820530) for treatment-naïve patients, led to the US Food and Drug Administration approval of iptacopan in December 2023 for all adults with PNH.
Stakeholders anticipate the widescale adoption of oral complement inhibitors such as iptacopan, citing the improvement in clinical efficacy, convenience, and decreased disease burden for individuals living with PNH. However, literature assessing the cost-effectiveness of this expensive new therapeutic in the care of either anti-C5–treated or treatment-naïve adults with PNH is limited to 1 study showing that iptacopan is not cost-effective.14 We sought to fill this gap by determining the cost-effectiveness of iptacopan vs the standard-of-care (SOC) for anti-C5–treated adults with PNH across societal and health system perspectives in 3 geographically and socioeconomically diverse jurisdictions (United States, Brazil, and Japan) in which anti-C5 treatments are approved.
Methods
Model overview
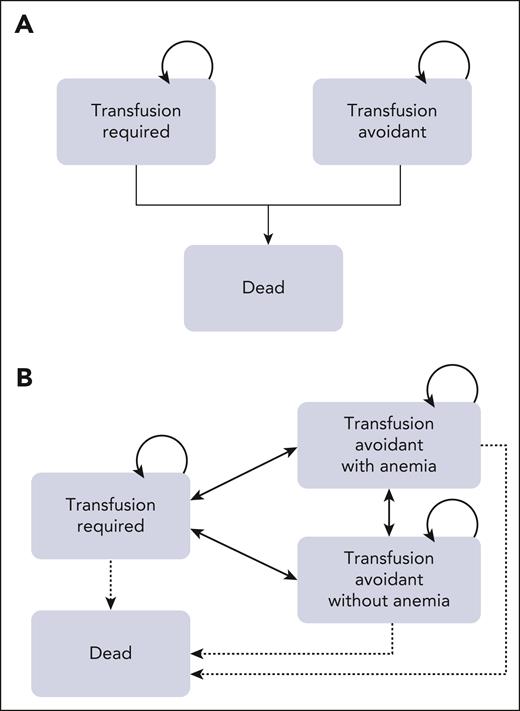
We built a base-case Markov cohort model to evaluate the cost-effectiveness of iptacopan monotherapy compared with the SOC treatment with C5 inhibition (ie, eculizumab and/or ravulizumab). Our model was based on the clinical data from APPLY-PNH, the open-label, randomized, phase 3 trial that examined the efficacy of oral iptacopan therapy in patients with PNH with residual anemia despite SOC with anti-C5 therapy.13 In our model, anti-C5–treated patients with PNH were assigned to 1 of 2 treatment arms paralleling the trial: oral iptacopan monotherapy or ongoing SOC with anti-C5 therapy (Figure 1). Patients in each arm entered the model at a median age of 51 years, with 69% being of female sex to reflect patient characteristics in APPLY-PNH.13 In the iptacopan arm, patients received 200 mg of oral iptacopan twice daily, following APPLY-PNH’s protocol.13 Reflecting trial baseline characteristics (as well as current treatment patterns) in the SOC arm in our model, 66% of patients received eculizumab and 34% of patients received ravulizumab.13,15,16 Patients received 900 mg of eculizumab every 2 weeks or 3300 mg of ravulizumab every 8 weeks IV, following the US Food and Drug Administration package insert and based on the trial population’s baseline characteristics.13 The cycle length of our model was 1 month, chosen to both reflect the clinical tempo of the disease and to be consistent with prior cost-effectiveness analyses in the PNH arena.17,18 In the base-case model, we assumed that patients stayed in their initial health states (ie, transfusion-required or transfusion-avoidant states) through the model time-horizon and compared model output with an additional model structure that incorporated adjusted, aggregated individual-level trial-specific patient transition probability matrix, allowing for the transition of individual patients between different health states (Figure 1A-B).14,19 In each cycle, patients in both arms could experience breakthrough hemolysis (BTH) and transfusion-related adverse events. Patients had risks of death because of transfusion-related adverse events and, separately, because of background mortality. Although severe adverse events including major adverse vascular events and infection were reported in a few cases, none of them resulted in treatment discontinuation or death during the 24-week observation period.13 Therefore, in our model, treatment-associated complications did not result in treatment discontinuation. We conducted the analysis over a lifetime time-horizon across the range of accepted willingness-to-pay (WTP) thresholds in the United States.14 TreeAge Pro Healthcare 2024 (TreeAge Software, Williamstown, MA) was used to build the model and conduct the analysis. The Consolidated Health Economic Evaluation Reporting Standards reporting guideline was implemented as applicable.
Model schematics. The base-case Markov model (A) consists of 3 health states, including transfusion required, transfusion avoidant, and death. Patients with residual anemia despite complement inhibition enter a transfusion-required state or transfusion-avoidant state in the very first cycle (ie, by the end of 1 month), reflecting their response (or lack thereof) as in the clinical trial. Efficacy (lack of transition from transfusion avoidant to transfusion required and vice versa) is informed by trial results. Patients could remain in these states throughout their lifetime or move to death at any point, due to either transfusion-associated mortality or background mortality. A scenario Markov model (B) consists of 4 health states, including transfusion required, transfusion avoidant (with and without anemia), and death: patients transition across 3 health states as informed by a transition probability matrix derived from adjusted and aggregated individual-level patient data from APPLY-PNH.
Model schematics. The base-case Markov model (A) consists of 3 health states, including transfusion required, transfusion avoidant, and death. Patients with residual anemia despite complement inhibition enter a transfusion-required state or transfusion-avoidant state in the very first cycle (ie, by the end of 1 month), reflecting their response (or lack thereof) as in the clinical trial. Efficacy (lack of transition from transfusion avoidant to transfusion required and vice versa) is informed by trial results. Patients could remain in these states throughout their lifetime or move to death at any point, due to either transfusion-associated mortality or background mortality. A scenario Markov model (B) consists of 4 health states, including transfusion required, transfusion avoidant (with and without anemia), and death: patients transition across 3 health states as informed by a transition probability matrix derived from adjusted and aggregated individual-level patient data from APPLY-PNH.
Transition probabilities
Base-case estimates and ranges for all input parameters used in our model are summarized in Table 1. Transfusion avoidance was achieved in 94.8% of patients treated with iptacopan and 25.9% of patients treated with SOC, matching the final results of APPLY-PNH.13 The number of red blood cell products required for transfusion-dependent patients was informed by the number of transfusions for patients in the SOC arm in the trial at baseline.13 Patients receiving transfusions could experience major transfusion-associated adverse events including delayed hemolytic transfusion reaction, transfusion-associated circulatory overload, and transfusion-related acute lung injury. Probabilities of incidence and death for these adverse events were obtained from hemovigilance reporting in the 2015 National Blood Collection and Utilization survey and published literature.20-23 The probabilities of BTH for the iptacopan arm and SOC arm were informed by the results from the iptacopan arm and SOC arm in APPLY-PNH, respectively.13 US Life Tables were used for age- and sex-specific background mortality.24
Base-case and scenario analysis model input parameters and distributions
| . | Base case . | Distributions . | References . |
|---|---|---|---|
| Clinical parameters | |||
| Age, y | 51 | 13 | |
| Eculizumab use in SOC, % | 66 | 13 | |
| Transfusion avoidance in patients treated with iptacopan, % | 94.8 | PERT (88.1-100.0) | 13 |
| Transfusion avoidance in patients treated with SOC, % | 25.9 | PERT (11.6-42.4) | 13 |
| Monthly probability of BTH in iptacopan | 0.0083 | PERT (0-0.0247) | 13 |
| Monthly probability of BTH in SOC | 0.0567 | PERT (0.0247-0.132) | 13 |
| Number of RBC units in transfusion-dependent patients in 6 mo | 4 | Gamma (0.89, 7.62) | 13 |
| Number of RBC units for BTH treatment | 2 | Gamma (4, 0.5) | 13 |
| Annual DHTR incidence, per product | 0.0000526 | PERT (0.0000421-0.0000632) | 20 |
| Annual TRALI incidence, per product | 0.0000175 | PERT (0.0000140-0.0000211) | 20 |
| Annual TACO incidence, per product | 0.000111 | PERT (0.0000889-0.000133) | 20 |
| DHTR mortality | 0.038 | PERT (0-0.064) | 21 |
| TRALI mortality | 0.075 | PERT (0.06-0.09) | 22 |
| TACO mortality | 0.084 | PERT (0.067-0.10) | 23 |
| Utilities | |||
| Transfusion independent (Hb ≥ 10.5 g/dL) | 0.808 | PERT (0.646-0.970) | 17 |
| Transfusion dependent | 0.695 | PERT (0.556-0.834) | 17 |
| Transfusion independent (PRINCE) | 0.869 | PERT (0.695-1) | 25 |
| Transfusion dependent (PRINCE) | 0.818 | PERT (0.556-0.799) | 25 |
| General population utility (female-to-male sex ratio (%), 69:31 | Age dependent | 26 | |
| Moderate anemia disutility | 0.052 | PERT (0.034-0.076) | 27 |
| Utility increment by oral therapy vs injection | 0.01 | PERT (0.008-0.012) | 28,29 |
| DHTR disutility | 0.4 | PERT (0.32-0.48) | 27 |
| TRALI disutility | 0.4 | PERT (0.32-0.48) | 30 |
| TACO disutility | 0.4 | PERT (0.32-0.48) | Assumption |
| BTH disutility | 0.4 | PERT (0.32-0.48) | 31 |
| Costs | |||
| US cost ($) | |||
| Iptacopan, per mg | 3.34 | 32 | |
| Eculizumab, per mg | 22.70 | 33 | |
| Ravulizumab, per mg | 22.27 | 33 | |
| Iptacopan, per mg (AWP) | 4.52 | 34 | |
| Eculizumab, per mg (AWP) | 26.09 | 34 | |
| Ravulizumab, per mg (AWP) | 25.62 | 34 | |
| Cost of administration | 160.63 | Gamma (100, 1.61) | 35 |
| RBC unit cost | 196.20 | Gamma (100, 1.96) | 36 |
| Transfusion administration | 1 240 | Gamma (100, 12.4) | 36 |
| Cost of DHTR | 1 537 | Gamma (100, 15.4) | 37 |
| Cost of TRALI | 9 957 | Gamma (100, 99.6) | 37 |
| Cost of TACO | 4 830 | Gamma (100, 48.3) | 37 |
| All-cause direct medical cost due to transfusion dependent (PPPY) | 252 010 | Gamma (100, 2520) | 38 |
| All-cause medical related absenteeism cost due to transfusion dependent (PPPY) | 4 935 | Gamma (100, 49.4) | 38 |
| Mean hourly wage (all occupation) | 29.76 | Gamma (100, 0.30) | 39 |
| Mean hourly wage (registered nurse) | 42.80 | Gamma (100, 0.43) | 39 |
| Japanese cost (¥) | |||
| Eculizumab (300 mg/30 mL) | 619 834 | 40 | |
| Ravulizumab (300 mg/3 mL) | 699 570 | 40 | |
| Eculizumab administration cost | 7 856 | 41 | |
| Ravulizumab administration cost | 11 761 | 41 | |
| RBC unit cost | 9 067 | 40 | |
| Transfusion administration | 4 500 | 42 | |
| Absenteeism cost | 387 345 | Gamma (100, 3 873) | 38 |
| Mean hourly wage (all employees) | 2 336 | Gamma (100, 23.4) | 43 |
| Mean hourly wage (nurse) | 2 633 | Gamma (100, 26.3) | 43 |
| Brazilian cost (R$) | |||
| Eculizumab (300 mg/30 mL) | 19 016.68 | 44 | |
| Ravulizumab (300 mg/3 mL) | 20 540.12 | 44 | |
| Administration cost | 0.63 | 18 | |
| RBC unit cost | 148.80 | 45 | |
| Transfusion administration | 8.09 | 18,46 | |
| Absenteeism cost | 4635 | Gamma (100, 46.4) | 38 |
| Mean hourly wage (all employees) | 27.95 | Gamma (100, 0.28) | 47 |
| Mean hourly wage (nurse) | 33.50 | Gamma (100, 0.34) | 48,49 |
| . | Base case . | Distributions . | References . |
|---|---|---|---|
| Clinical parameters | |||
| Age, y | 51 | 13 | |
| Eculizumab use in SOC, % | 66 | 13 | |
| Transfusion avoidance in patients treated with iptacopan, % | 94.8 | PERT (88.1-100.0) | 13 |
| Transfusion avoidance in patients treated with SOC, % | 25.9 | PERT (11.6-42.4) | 13 |
| Monthly probability of BTH in iptacopan | 0.0083 | PERT (0-0.0247) | 13 |
| Monthly probability of BTH in SOC | 0.0567 | PERT (0.0247-0.132) | 13 |
| Number of RBC units in transfusion-dependent patients in 6 mo | 4 | Gamma (0.89, 7.62) | 13 |
| Number of RBC units for BTH treatment | 2 | Gamma (4, 0.5) | 13 |
| Annual DHTR incidence, per product | 0.0000526 | PERT (0.0000421-0.0000632) | 20 |
| Annual TRALI incidence, per product | 0.0000175 | PERT (0.0000140-0.0000211) | 20 |
| Annual TACO incidence, per product | 0.000111 | PERT (0.0000889-0.000133) | 20 |
| DHTR mortality | 0.038 | PERT (0-0.064) | 21 |
| TRALI mortality | 0.075 | PERT (0.06-0.09) | 22 |
| TACO mortality | 0.084 | PERT (0.067-0.10) | 23 |
| Utilities | |||
| Transfusion independent (Hb ≥ 10.5 g/dL) | 0.808 | PERT (0.646-0.970) | 17 |
| Transfusion dependent | 0.695 | PERT (0.556-0.834) | 17 |
| Transfusion independent (PRINCE) | 0.869 | PERT (0.695-1) | 25 |
| Transfusion dependent (PRINCE) | 0.818 | PERT (0.556-0.799) | 25 |
| General population utility (female-to-male sex ratio (%), 69:31 | Age dependent | 26 | |
| Moderate anemia disutility | 0.052 | PERT (0.034-0.076) | 27 |
| Utility increment by oral therapy vs injection | 0.01 | PERT (0.008-0.012) | 28,29 |
| DHTR disutility | 0.4 | PERT (0.32-0.48) | 27 |
| TRALI disutility | 0.4 | PERT (0.32-0.48) | 30 |
| TACO disutility | 0.4 | PERT (0.32-0.48) | Assumption |
| BTH disutility | 0.4 | PERT (0.32-0.48) | 31 |
| Costs | |||
| US cost ($) | |||
| Iptacopan, per mg | 3.34 | 32 | |
| Eculizumab, per mg | 22.70 | 33 | |
| Ravulizumab, per mg | 22.27 | 33 | |
| Iptacopan, per mg (AWP) | 4.52 | 34 | |
| Eculizumab, per mg (AWP) | 26.09 | 34 | |
| Ravulizumab, per mg (AWP) | 25.62 | 34 | |
| Cost of administration | 160.63 | Gamma (100, 1.61) | 35 |
| RBC unit cost | 196.20 | Gamma (100, 1.96) | 36 |
| Transfusion administration | 1 240 | Gamma (100, 12.4) | 36 |
| Cost of DHTR | 1 537 | Gamma (100, 15.4) | 37 |
| Cost of TRALI | 9 957 | Gamma (100, 99.6) | 37 |
| Cost of TACO | 4 830 | Gamma (100, 48.3) | 37 |
| All-cause direct medical cost due to transfusion dependent (PPPY) | 252 010 | Gamma (100, 2520) | 38 |
| All-cause medical related absenteeism cost due to transfusion dependent (PPPY) | 4 935 | Gamma (100, 49.4) | 38 |
| Mean hourly wage (all occupation) | 29.76 | Gamma (100, 0.30) | 39 |
| Mean hourly wage (registered nurse) | 42.80 | Gamma (100, 0.43) | 39 |
| Japanese cost (¥) | |||
| Eculizumab (300 mg/30 mL) | 619 834 | 40 | |
| Ravulizumab (300 mg/3 mL) | 699 570 | 40 | |
| Eculizumab administration cost | 7 856 | 41 | |
| Ravulizumab administration cost | 11 761 | 41 | |
| RBC unit cost | 9 067 | 40 | |
| Transfusion administration | 4 500 | 42 | |
| Absenteeism cost | 387 345 | Gamma (100, 3 873) | 38 |
| Mean hourly wage (all employees) | 2 336 | Gamma (100, 23.4) | 43 |
| Mean hourly wage (nurse) | 2 633 | Gamma (100, 26.3) | 43 |
| Brazilian cost (R$) | |||
| Eculizumab (300 mg/30 mL) | 19 016.68 | 44 | |
| Ravulizumab (300 mg/3 mL) | 20 540.12 | 44 | |
| Administration cost | 0.63 | 18 | |
| RBC unit cost | 148.80 | 45 | |
| Transfusion administration | 8.09 | 18,46 | |
| Absenteeism cost | 4635 | Gamma (100, 46.4) | 38 |
| Mean hourly wage (all employees) | 27.95 | Gamma (100, 0.28) | 47 |
| Mean hourly wage (nurse) | 33.50 | Gamma (100, 0.34) | 48,49 |
AWP, average wholesale price; DHTR, delayed hemolytic transfusion reaction; Hb, hemoglobin; PERT, project evaluation and review techniques; PPPY, per person per year; RBC, red blood cell; TACO, transfusion-associated circulatory overload; TRALI, transfusion-related acute lung injury.
Costs
Costs were assessed from the societal and health care system perspectives. All costs were estimated in 2023 US dollars using the medical care component of the Consumer Price Index.50 The iptacopan price of $488 220 per year was obtained from the Medicare Part D price list.32 The prices of eculizumab and ravulizumab were obtained from the 2023 Centers for Medicare & Medicaid Services (CMS).33 Although Medicaid and Medicare–insured patients are under the auspices of the single largest insurer in the United States, covering ∼37% of the population, we separately conducted a scenario analysis in the more heterogeneous commercial insurance infrastructure using average wholesale price. Costs of transfusion care, BTH, and direct and indirect medical costs are in supplemental Data, available on the Blood website.
QALYs
Health outcomes were calculated in quality-adjusted life-years (QALYs), a measure that accounts for both health-related quality of life and length of life. Health-related quality of life was quantified by utility values, which range from 0 (death) to 1 (perfect health). In the absence of utility values reported in APPLY-PNH, we used the PNH-specific utility values from a prior cost-effectiveness analysis.17 These values were calculated using the Longworth mapping method with the European Organization for Research and Treatment of Cancer Quality of Life Questionnaire-Core 30 Scale score from the PEGASUS study (ClinicalTrials.gov identifier: NCT03500549), allowing for specific utility values for patients with PNH who receive transfusions (utility 0.695) vs transfusion independence (utility 0.808).10,51 We multiplicatively incorporated an age-dependent baseline utility based on the 5-level EQ-5D (EQ-5D-5L) for the general population of the United States.26 The age-, sex-dependent EQ-5D-5L score for the US general population was weighted to match the APPLY-PNH trial population, and then multiplied by these PNH-specific utility weights. Beyond testing this across extensive sensitivity analyses (see “Cost-effectiveness, threshold, scenario, and sensitivity analyses”), we additionally evaluated 2 separate scenario analyses using different utility values. In the first, we used utility values obtained from the PRINCE study (ClinicalTrials.gov identifier: NCT04085601), which reported a more conservative increment from 0.818 for patients requiring transfusion to 0.869 for transfusion-independent patients with PNH.25,52 In the second, we report a scenario analysis using the Global Burden of Disease database to account for the disutility avoided across the trial patient population by the complete correction of moderate anemia at baseline in APPLY-PNH with iptacopan use (vs persistence of moderate anemia with ongoing SOC).26,27 In all cases (base-case and scenario analyses), disutilities due to transfusion-related adverse events and BTH were informed by prior literature.27,30,31 We considered also the utility increment seen with the route of administration (ie, oral therapy compared with injection and infusion therapies). A previous health-related quality-of-life study in type 2 diabetes mellitus comparing oral and once-weekly injection administration reported a utility increment of 0.01.28 Similarly, when comparing oral antibiotics with injectable forms, a utility increase of 0.008 was reported.29 Although there are no studies comparing the utility of oral therapy with IV infusion at 2-week or 8-week intervals, based on prior literature, we used a utility increment of oral iptacopan therapy compared with SOC of 0.01 in a base-case analysis, evaluating the effect of this assumption in sensitivity analyses.
Cost-effectiveness, threshold, scenario, and sensitivity analyses
We performed the cost-effectiveness analysis from the US societal and health care system perspectives. Our analysis was conducted over a lifetime time-horizon across accepted WTP thresholds of $50 000, $100 000, and $150 000 per QALY in the United States. Both cost and health outcomes were discounted by 3% annually.53,54 The 2 primary outcomes for iptacopan monotherapy in this analysis were (1) the incremental cost-effectiveness ratio or the incremental net monetary benefit (iNMB), if the therapy was found to be cost saving at a WTP threshold of $150 000 per QALY; and (2) the cost-effective maximum monthly threshold price of iptacopan monotherapy, as compared with the SOC. The secondary outcome was time saved for patients and nurses with the use of oral iptacopan therapy (as compared with that of IV eculizumab or ravulizumab therapy). We also performed 6 additional scenario analyses: (1) short-run time horizon of 5 years (considering that the long-term efficacy and safety of iptacopan beyond 48 weeks are still unclear); (2) utility weights as informed by the PRINCE trial25,52; (3) utility weights as informed by the degree of hemoglobin improvement (ie, a primary outcome of the APPLY-PNH trial)27; (4) average wholesale price; (5) treatment discontinuation (lifetime and 5-year time horizons); and (6) incorporating individual-level trial-specific patient transition probability matrix and health state utility values used by National Institute for Health and Care Excellence (NICE) (see supplemental Tables 1 and 2).19 For the treatment discontinuation scenario, annual probability of iptacopan discontinuation (back to anti-C5 therapy) was informed by APPLY-PNH, which reported 1 patient switching because of pregnancy.13 Given that there was 1 discontinuation in 28.66 patient-years of follow-up, the discontinuation probability was calculated to be 3.43% per year. We further evaluated this with a threshold analysis for the maximum annual discontinuation probability for which iptacopan became cost-ineffective. In addition, we separately evaluated both extremes of C5 inhibitor prevalent use mix, reporting our data as compared with each of 100% eculizumab users, 100% ravulizumab users, and the entire C5 inhibitor fractional use mix in between. Additionally, a 2-way sensitivity analysis on health care resource utilization cost and SOC efficacy was conducted. Deterministic and probabilistic sensitivity analyses are described in supplemental Data.
Global perspectives: Japan and Brazil
After a careful review of marketing authorizations in which at least 1 complement inhibitor is approved in the care of people living with PNH, we sourced country-specific data to present dual base-case (ie, societal and health system) perspectives from 2 additional jurisdictions: Japan and Brazil. We chose these countries to ensure both geographic and socioeconomic diversity in our analysis. Given that iptacopan was first approved in the United States and price points are yet to be set in Japan and Brazil (first pending approval in the latter), we present cost-effectiveness results nonnormatively: as thresholds to estimate the maximum cost-effective price of iptacopan, broken down by C5 inhibitor and by short- and long-run time horizons. Detailed input parameters and methods for the Japanese and Brazilian analyses are available in the supplemental Data.
Results
Base-case and scenario analyses
The results of our model are summarized in Table 2. In the base case, at the annual iptacopan (CMS) price of $488 220, iptacopan monotherapy vs anti-C5 therapy accrued 12.6 and 10.8 QALYs at costs of $9.52 million and $13.5 million, respectively. The iNMB of iptacopan monotherapy over anti-C5 therapy at the WTP threshold of $150 000 per QALY was $4 280 000 (95% credible interval, 3 390 000-5 210 000). The breakdown of lifetime costs is presented in supplemental Table 3. Iptacopan vs anti-C5 therapy projects to save patients and nurses ∼730 hours (ravulizumab) and 2920 hours (eculizumab) over a lifetime in PNH-specific care averted.
Base-case and scenario analysis results for iptacopan monotherapy vs anticomplement C5 therapy
| Strategy . | Cost (million $) . | Incremental cost (million $) . | Effectiveness (QALYs) . | Incremental effectiveness (QALYs) . | % cost-effective at WTP threshold of $50 000 per QALY . | % cost-effective at WTP threshold of $100 000 per QALY . | % cost-effective at WTP threshold of $150 000 per QALY . |
|---|---|---|---|---|---|---|---|
| US societal perspective | |||||||
| Base case∗ | |||||||
| Iptacopan | 9.52 | 12.6 | 100 | 100 | 100 | ||
| Anti-C5 | 13.5 | 4.01 | 10.8 | −1.78 | 0 | 0 | 0 |
| iNMB at WTP thresholds of $50 000, $100 000, $150 000 per QALY (95% CI) $4 100 000 (3 250 000-4 990 000), $4 190 000 (3 330 000-5 100 000), $4 280 000 (3 390 000-5 210 000). | |||||||
| Scenario (5-y time horizon) | |||||||
| Iptacopan | 2.34 | 3.08 | 100 | 100 | 100 | ||
| Anti-C5 | 3.33 | 0.99 | 2.65 | −0.43 | 0 | 0 | 0 |
| iNMB at WTP thresholds of $50 000, $100 000, $150 000 per QALY (95% CI) $1 010 000 (803 000-1 230 000), $1 030 000 (822 000-1 260 000), $1 050 000 (837 000-1 290 000) | |||||||
| Scenario (PRINCE utilities) | |||||||
| Iptacopan | 9.52 | 13.6 | 100 | 100 | 100 | ||
| Anti-C5 | 13.5 | 4.01 | 12.5 | −1.11 | 0 | 0 | 0 |
| iNMB at WTP thresholds of $50 000, $100 000, $150 000 per QALY (95% CI) $4 070 000 (3 240 000-4 960 000), $4 120 000 (3 290 000-5 030 000), $4 180 000 (3 340 000-5 120 000) | |||||||
| Scenario (GBD 2019 utilities) | |||||||
| Iptacopan | 9.52 | 15.7 | 100 | 100 | 100 | ||
| Anti-C5 | 13.5 | 4.01 | 14.3 | −1.38 | 0 | 0 | 0 |
| iNMB at WTP thresholds of $50 000, $100 000, $150 000 per QALY (95% CI) $4 080 000 (3 250 000-4 960 000), $4 150 000 (3 320 000-5 040 000), $4 220 000 (3 390 000-5 110 000) | |||||||
| Scenario (average wholesale price) | |||||||
| Iptacopan | 12.8 | 12.6 | 100 | 100 | 100 | ||
| Anti-C5 | 15.0 | 2.21 | 10.8 | −1.78 | 0 | 0 | 0 |
| iNMB at WTP thresholds of $50 000, $100 000, $150 000 per QALY (95% CI) $2 300 000 (1 450 000-3 180 000), $2 390 000 (1 530 000-3 290 000), $2 480 000 (1 590 000-3 410 000) | |||||||
| Scenario (iptacopan discontinuation) | |||||||
| Iptacopan | 11.1 | 11.8 | 100 | 100 | 100 | ||
| Anti-C5 | 13.5 | 2.48 | 10.9 | −1.02 | 0 | 0 | 0 |
| iNMB at WTP thresholds of $50 000, $100 000, $150 000 per QALY (95% CI) $2 530 000 (1 890 000-3 190 000), $2 580 000 (1 920 000-3 260 000), $2 630 000 (1 960 000-3 330 000) | |||||||
| Scenario (Iptacopan discontinuation, 5-y time horizon) | |||||||
| Iptacopan | 2.43 | 3.04 | 100 | 100 | 100 | ||
| Anti-C5 | 3.33 | 0.90 | 2.65 | −0.39 | 0 | 0 | 0 |
| iNMB at WTP thresholds of $50 000, $100 000, $150 000 per QALY (95% CI) $920 000 (726 000-1 130 000), $940 000 (741 000-1 150 000), $959 000 (756 000-1 180 000) | |||||||
| Scenario (individual-level, trial-specific patient data transition probability matrix) | |||||||
| Iptacopan | 9.28 | 12.6 | 100 | 100 | 100 | ||
| Anti-C5 | 11.7 | 2.38 | 10.9 | −1.70 | 0 | 0 | 0 |
| iNMB at WTP thresholds of $50 000, $100 000, $150 000 per QALY (95% CI) $2 470 000 (2 150 000-2 810 000), $2 550 000 (2 230 000-2 910 000), $2 640 000 (2 320 000-3 000 000) | |||||||
| Scenario (individual-level, trial-specific patient data transition probability matrix and health state utilities) | |||||||
| Iptacopan | 9.28 | 13.7 | 100 | 100 | 100 | ||
| Anti-C5 | 11.7 | 2.38 | 10.9 | −2.83 | 0 | 0 | 0 |
| iNMB at WTP thresholds $50 000, $100 000, $150 000 per QALY (95% CI) $2 520 000 (2 200 000-2 860 000), $2 660 000 (2 330 000-3 010 000), $2 800 000 (2 480 000-3 170 000) | |||||||
| US health care system perspective | |||||||
| Base case | |||||||
| Iptacopan | 9.52 | 12.6 | 100 | 100 | 100 | ||
| Anti-C5 | 13.4 | 3.84 | 10.8 | −1.78 | 0 | 0 | 0 |
| iNMB at WTP thresholds $50 000, $100 000, $150 000 per QALY (95% CI) $3 930 000 (3 090 000-4 810 000), $4 020 000 (3 170 000-4 920 000), $4 110 000 (3 230 000-5 040 000) | |||||||
| Scenario (5-y time horizon) | |||||||
| Iptacopan | 2.34 | 3.08 | 100 | 100 | 100 | ||
| Anti-C5 | 3.29 | 0.95 | 2.65 | −0.43 | 0 | 0 | 0 |
| iNMB at WTP thresholds of $50 000, $100 000, $150 000 per QALY (95% CI) $969 000 (760 000-1 190 000), $991 000 (781 000-1 210 000), $1 010 000 (797 000-1 240 000) | |||||||
| Strategy . | Cost (million $) . | Incremental cost (million $) . | Effectiveness (QALYs) . | Incremental effectiveness (QALYs) . | % cost-effective at WTP threshold of $50 000 per QALY . | % cost-effective at WTP threshold of $100 000 per QALY . | % cost-effective at WTP threshold of $150 000 per QALY . |
|---|---|---|---|---|---|---|---|
| US societal perspective | |||||||
| Base case∗ | |||||||
| Iptacopan | 9.52 | 12.6 | 100 | 100 | 100 | ||
| Anti-C5 | 13.5 | 4.01 | 10.8 | −1.78 | 0 | 0 | 0 |
| iNMB at WTP thresholds of $50 000, $100 000, $150 000 per QALY (95% CI) $4 100 000 (3 250 000-4 990 000), $4 190 000 (3 330 000-5 100 000), $4 280 000 (3 390 000-5 210 000). | |||||||
| Scenario (5-y time horizon) | |||||||
| Iptacopan | 2.34 | 3.08 | 100 | 100 | 100 | ||
| Anti-C5 | 3.33 | 0.99 | 2.65 | −0.43 | 0 | 0 | 0 |
| iNMB at WTP thresholds of $50 000, $100 000, $150 000 per QALY (95% CI) $1 010 000 (803 000-1 230 000), $1 030 000 (822 000-1 260 000), $1 050 000 (837 000-1 290 000) | |||||||
| Scenario (PRINCE utilities) | |||||||
| Iptacopan | 9.52 | 13.6 | 100 | 100 | 100 | ||
| Anti-C5 | 13.5 | 4.01 | 12.5 | −1.11 | 0 | 0 | 0 |
| iNMB at WTP thresholds of $50 000, $100 000, $150 000 per QALY (95% CI) $4 070 000 (3 240 000-4 960 000), $4 120 000 (3 290 000-5 030 000), $4 180 000 (3 340 000-5 120 000) | |||||||
| Scenario (GBD 2019 utilities) | |||||||
| Iptacopan | 9.52 | 15.7 | 100 | 100 | 100 | ||
| Anti-C5 | 13.5 | 4.01 | 14.3 | −1.38 | 0 | 0 | 0 |
| iNMB at WTP thresholds of $50 000, $100 000, $150 000 per QALY (95% CI) $4 080 000 (3 250 000-4 960 000), $4 150 000 (3 320 000-5 040 000), $4 220 000 (3 390 000-5 110 000) | |||||||
| Scenario (average wholesale price) | |||||||
| Iptacopan | 12.8 | 12.6 | 100 | 100 | 100 | ||
| Anti-C5 | 15.0 | 2.21 | 10.8 | −1.78 | 0 | 0 | 0 |
| iNMB at WTP thresholds of $50 000, $100 000, $150 000 per QALY (95% CI) $2 300 000 (1 450 000-3 180 000), $2 390 000 (1 530 000-3 290 000), $2 480 000 (1 590 000-3 410 000) | |||||||
| Scenario (iptacopan discontinuation) | |||||||
| Iptacopan | 11.1 | 11.8 | 100 | 100 | 100 | ||
| Anti-C5 | 13.5 | 2.48 | 10.9 | −1.02 | 0 | 0 | 0 |
| iNMB at WTP thresholds of $50 000, $100 000, $150 000 per QALY (95% CI) $2 530 000 (1 890 000-3 190 000), $2 580 000 (1 920 000-3 260 000), $2 630 000 (1 960 000-3 330 000) | |||||||
| Scenario (Iptacopan discontinuation, 5-y time horizon) | |||||||
| Iptacopan | 2.43 | 3.04 | 100 | 100 | 100 | ||
| Anti-C5 | 3.33 | 0.90 | 2.65 | −0.39 | 0 | 0 | 0 |
| iNMB at WTP thresholds of $50 000, $100 000, $150 000 per QALY (95% CI) $920 000 (726 000-1 130 000), $940 000 (741 000-1 150 000), $959 000 (756 000-1 180 000) | |||||||
| Scenario (individual-level, trial-specific patient data transition probability matrix) | |||||||
| Iptacopan | 9.28 | 12.6 | 100 | 100 | 100 | ||
| Anti-C5 | 11.7 | 2.38 | 10.9 | −1.70 | 0 | 0 | 0 |
| iNMB at WTP thresholds of $50 000, $100 000, $150 000 per QALY (95% CI) $2 470 000 (2 150 000-2 810 000), $2 550 000 (2 230 000-2 910 000), $2 640 000 (2 320 000-3 000 000) | |||||||
| Scenario (individual-level, trial-specific patient data transition probability matrix and health state utilities) | |||||||
| Iptacopan | 9.28 | 13.7 | 100 | 100 | 100 | ||
| Anti-C5 | 11.7 | 2.38 | 10.9 | −2.83 | 0 | 0 | 0 |
| iNMB at WTP thresholds $50 000, $100 000, $150 000 per QALY (95% CI) $2 520 000 (2 200 000-2 860 000), $2 660 000 (2 330 000-3 010 000), $2 800 000 (2 480 000-3 170 000) | |||||||
| US health care system perspective | |||||||
| Base case | |||||||
| Iptacopan | 9.52 | 12.6 | 100 | 100 | 100 | ||
| Anti-C5 | 13.4 | 3.84 | 10.8 | −1.78 | 0 | 0 | 0 |
| iNMB at WTP thresholds $50 000, $100 000, $150 000 per QALY (95% CI) $3 930 000 (3 090 000-4 810 000), $4 020 000 (3 170 000-4 920 000), $4 110 000 (3 230 000-5 040 000) | |||||||
| Scenario (5-y time horizon) | |||||||
| Iptacopan | 2.34 | 3.08 | 100 | 100 | 100 | ||
| Anti-C5 | 3.29 | 0.95 | 2.65 | −0.43 | 0 | 0 | 0 |
| iNMB at WTP thresholds of $50 000, $100 000, $150 000 per QALY (95% CI) $969 000 (760 000-1 190 000), $991 000 (781 000-1 210 000), $1 010 000 (797 000-1 240 000) | |||||||
Iptacopan monotherapy is a cost-saving treatment option across all scenarios.
CI, credible interval; iNMB, incremental net monetary benefit; QALY, quality-adjusted life year; WTP, willingness-to-pay.
Patient time lost from C5 inhibitor use in base case: 730 hours from ravulizumab and 2920 hours from eculizumab.
In the scenario analysis with a 5-year time horizon, iptacopan monotherapy vs anti-C5 therapy accrued 3.08 and 2.65 QALYs at costs of $2.34 million and $3.33 million, respectively. The iNMB of iptacopan monotherapy over anti-C5 therapy was $1 050 000 (95% credible interval, 837 000-1 290 000) at a WTP threshold of $150 000 per QALY. In the scenario analysis using utility values from the PRINCE trial, iptacopan monotherapy vs anti-C5 therapy accrued 13.6 and 12.5 QALYs at costs of $9.52 million and $13.5 million, respectively. The iNMB of iptacopan monotherapy over anti-C5 therapy was $4 180 000 (95% credible interval, 3 340 000-5 120 000) at a WTP threshold of $150 000 per QALY. Finally, in the scenario analysis using utility values from resolving anemia, iptacopan monotherapy vs anti-C5 therapy accrued 15.7 and 14.3 QALYs, respectively, with the iNMB of iptacopan monotherapy over anti-C5 therapy of $4 220 000 (95% credible interval, 3 390 000-5 110 000) at a WTP threshold of $150 000 per QALY. In the scenario considering the discontinuation of iptacopan therapy, iptacopan monotherapy accrued 11.8 QALYs at a cost of $11.1 million, with an iNMB of $2 630 000 over anti-C5 therapy (95% credible interval, 1 960 000-3 330 000) over lifetime time horizon and 3.04 QALYs at a cost of $2.43 million, with an iNMB of $959 000 (95% credible interval, 756 000-1 180 000) over a 5-year time horizon, at a WTP threshold of $150 000 per QALY. The threshold analysis showed that iptacopan became cost-ineffective at an annual probability of discontinuation of 36% (supplemental Figure 1).
Deterministic, probabilistic, and threshold sensitivity analyses
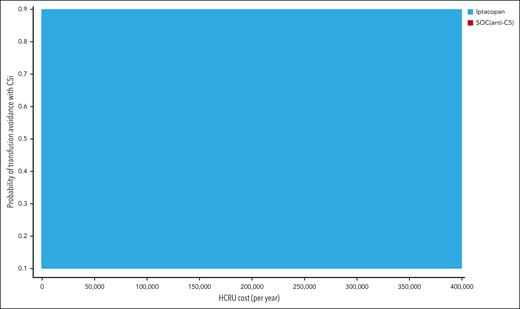
The results of 1-way deterministic sensitivity analysis are shown as a tornado diagram (Figure 2). Five parameters were identified as affecting the primary outcome (iNMB) at least 10% in either direction, with none changing the conclusion that iptacopan is a cost-saving therapeutic option. In descending order of priority, these were: (1) monthly cost of iptacopan, (2) monthly cost of eculizumab, (3) health care resource utilization costs of transfusion-dependent patients with PNH, (4) transfusion avoidance in SOC C5 inhibition, and (5) monthly cost of ravulizumab. The additional 1-way sensitivity analysis on the percentage of eculizumab users showed that the cost savings of iptacopan remain consistent across scenarios ranging from 100% ravulizumab use to 100% eculizumab use (supplemental Figure 2). Threshold analysis showed the maximum monthly price of iptacopan was $59 500, which corresponds to ∼146% of the Medicare Part D price. In the scenario with a 5-year time horizon considering the discontinuation of iptacopan therapy, the maximum monthly price for iptacopan to be cost-effective was $59 300 (146% of the Medicare Part D price). Across all probabilistic sensitivity analyses, iptacopan monotherapy was favored over the SOC C5 inhibition in 100% of the 10 000 Monte Carlo iterations (Table 2; Figure 3). The 2-way sensitivity analysis revealed iptacopan therapy to be cost saving over SOC at low health care–associated cost burdens and high SOC transfusion avoidance (Figure 4). Finally, iptacopan cost-threshold analyses stratified by C5 inhibitor across country, perspective, and time horizon noted thresholds of ∼1.4, 1.4, and 1.1, respectively, for iptacopan to be a cost-effective therapeutic choice in the United States, Japan, and Brazil, respectively (supplemental Table 4).
Tornado diagram: 1-way deterministic sensitivity analyses. Each row illustrates analytic outcome results (ie, change in iNMB) when each model parameter is varied across its range. Parameters affecting the iNMB at least 10% in either direction are shown. Blue bar denotes iNMB change with decreasing parameter values, and red bar denotes iNMB change with increasing parameter values. C5i, C5 inhibitor; Ecu, eculizumab; EV, expected value; HCRU, healthcare resource utilization; Rav, ravulizumab.
Tornado diagram: 1-way deterministic sensitivity analyses. Each row illustrates analytic outcome results (ie, change in iNMB) when each model parameter is varied across its range. Parameters affecting the iNMB at least 10% in either direction are shown. Blue bar denotes iNMB change with decreasing parameter values, and red bar denotes iNMB change with increasing parameter values. C5i, C5 inhibitor; Ecu, eculizumab; EV, expected value; HCRU, healthcare resource utilization; Rav, ravulizumab.
Cost-effectiveness acceptability curve of probabilistic sensitivity analysis for iptacopan vs SOC. The distribution of each parameter used in the analysis is detailed in Table 1. Across all conventionally accepted WTP thresholds in the United States (ie, $50 000-$150 000 per QALY) iptacopan monotherapy is favored over SOC in 100% of 10 000 iterations.
Cost-effectiveness acceptability curve of probabilistic sensitivity analysis for iptacopan vs SOC. The distribution of each parameter used in the analysis is detailed in Table 1. Across all conventionally accepted WTP thresholds in the United States (ie, $50 000-$150 000 per QALY) iptacopan monotherapy is favored over SOC in 100% of 10 000 iterations.
Two-way sensitivity analysis on HCRU cost and transfusion avoidance probability with anti-C5 therapy (ie, SOC). Iptacopan was cost saving over SOC C5 inhibitors at high rates of transfusion avoidance in anti-C5–treated patients receiving SOC and low HCRU costs.
Two-way sensitivity analysis on HCRU cost and transfusion avoidance probability with anti-C5 therapy (ie, SOC). Iptacopan was cost saving over SOC C5 inhibitors at high rates of transfusion avoidance in anti-C5–treated patients receiving SOC and low HCRU costs.
Discussion
We assessed the cost-effectiveness of oral iptacopan monotherapy compared with SOC C5 inhibition in anti-C5–treated adults living with PNH from the US societal and health care system perspectives. Our results across the base case, extensive sensitivity, and scenario analyses are consistent with the finding that iptacopan contributes to a higher quality of life for patients with PNH at a lower overall cost burden, as compared with C5 inhibitors.
The evolution of anticomplement therapy from IV antibodies to subcutaneous peptides, and now to oral small molecules, highlights the goal of providing patients with convenient and effective treatments. Subcutaneous pegcetacoplan allows for greater perceived independence of patients from the burden of IV infusions but it is limited by the cumbersome nature of pump-syringe systems and higher dosing frequencies.12,55 Moreover, for patients, this still entails an infusion time commitment, nurse-led training, and active self-adherence.17 Oral formulations generally promote better adherence and prescription fulfillment compared with injection or infusion-based systems.56 However, daily oral medications still pose a risk of missed doses.57 Specifically for iptacopan, missed dosing could potentially trigger BTH due to incomplete proximal complement inhibition, leading to C5-cascade–mediated intravascular hemolysis, although such events were not observed in the APPLY-PNH trial.11,13 For patients who struggle with twice daily iptacopan adherence, our results indicate that up to 36% of them could switch back to C5 inhibitor infusions, for iptacopan therapy to still be cost-effective from a societal perspective. Altogether, given the current unmet need and patient burden with nonoral therapeutics, oral complement inhibitors are poised to greatly benefit patients.58 The adoption of targeted, oral-based treatment systems has been witnessed from the use of tamoxifen for breast cancer adjuvant therapy, to the widespread use of direct oral anticoagulants for anticoagulation.59,60 Overall, such entries of oral treatment options have consistently been associated with rapid improvements in patients’ sense of control, ease of access, and overall quality of life.61-63 Therefore, for the treatment of PNH and other complement-based disorders, the adoption of oral complement inhibitors such as iptacopan follows an established and effective paradigm.
Our model and cost-saving findings for iptacopan extend the literature and are unique in several ways. A recent cost-effectiveness analysis conducted by the Institute for Clinical and Economic Review (ICER) indicated that iptacopan monotherapy was not cost-effective for anti-C5–treated patients compared with ravulizumab with an incremental cost-effectiveness ratio of $1 368 000 per QALY (this increased to ∼$11 million per QALY with cost offsetting).14 Using ICER’s input parameters, our model yields similar results. However, we reached a different conclusion than ICER because of several critical differences in input parameters (detailed in supplemental Table 5). First, we used and provide supportive evidence for the latest Medicare Part D annual price for iptacopan ($488 220), which is 11% below the wholesale acquisition price used by the ICER ($550 377).14,32 Second, despite the advantageous profile of ravulizumab and the ongoing use shift from eculizumab to ravulizumab, eculizumab is prescribed as the first line of treatment in 68% to 85% of patients, even in jurisdictions in which ravulizumab is approved, and therefore is relevant for comparison with iptacopan.15,16 Our study, therefore reflects the current mixed use of eculizumab and ravulizumab, including respective cost and health care resource burden (ie, accrued costs are lower with eculizumab than with ravulizumab), and, additionally, reports all results separately for eculizumab and for ravulizumab. Third, given the evolution of anticomplement agents toward patient convenience and with support from prior literature, we integrate a utility increment specific to the availability of oral therapy. Fourth, we capture the overall health care resource burden attributed to transfusion dependency in PNH by using data across hospitalizations, complications, and office visits. This further emphasizes the goal of providing health care resource-conscious treatments. Fifth, our model includes adverse events associated with transfusion dependency, including transfusion-related acute lung injury, transfusion-associated circulatory overload, and delayed hemolytic transfusion reaction, important contributors to mortality and decreased quality of life. Altogether, we believe these differences provide a more accurate, patient-centered, and society-relevant cost-effectiveness assessment of iptacopan monotherapy for anti-C5–treated patients with PNH. Furthermore, our findings are supported by scenario analysis that incorporates aggregated and adjusted trial-specific individual-level data and expands our model structure to reflect what was presented to NICE. Explicit comparisons to ICER and NICE, as well as extensive sensitivity and scenario analyses, all support the dual base-case findings for iptacopan cost-effectiveness in anti-C5–treated patients.
Our findings must be carefully interpreted within the existing pricing landscape of PNH. In the past decade, anti-C5 therapy itself has not been found to be cost-effective compared with SOC in the precomplement inhibition era (ie, treatment with anticoagulation and best supportive care) in the Brazilian and Canadian contexts.14,18,64 This was mainly attributed to the elevated prices of the C5 inhibitors themselves. Despite this, C5 inhibitors were adopted for widespread use, and remain a core component of PNH treatment. Critically, given the cost-ineffective baseline of C5 inhibitors themselves, our study does not support incrementally increased prices for newer PNH medications in proportion purely to incremental QALYs achieved as compared with the prior SOC (ie, C5 inhibitors). Such practice could contribute to misaligned incentives in pharmaceutical development and further undermine fair pricing. Concerns about unsustainable pricing (for society) relative to actual cost accrued in research, development, and manufacture of therapeutics has repeatedly led health technology assessment agencies to request details on actual costs, including in the PNH setting.64,65 The ICER noted that a fair price assessment for iptacopan should be subject to cost-offset capping given the high prices of C5 inhibitors in the United States. Cost-offset capping is a cost-effectiveness analysis method that transparently and effectively reprices the SOC, given commonly accepted costs per QALY to more accurately assess the value of a therapeutic (supplemental Table 6).66 This is relevant when the cumulative discounted cost of the iptacopan strategy is higher than that of SOC, as reported in the ICER report, but the reported lower CMS cost of iptacopan lends to a lower cumulative discounted cost of the iptacopan strategy. Because C5 inhibitor use has been the baseline SOC for more than a decade in the high-income country context, owing to literature at the time reporting an increased mortality hazard without anti-C5 therapy,67-70 replacing the current SOC with iptacopan at the current CMS pricing would indeed not incur increased cost but rather produce cost savings. The current price of iptacopan is critical to this analysis and is a stark reminder of the importance of value-informed pricing for the health of our societies (ie, health opportunity costs forgone).
Globally, the cost-effectiveness of iptacopan is uncertain, with market approval and adoption pending in numerous countries. Our iptacopan price thresholds for Brazil and Japan, from both health care system and societal perspectives, expand the scope of our study beyond the United States’ perspective and provide benchmarks for iptacopan’s cost-effectiveness over C5 inhibitors in anti-C5–treated patients in these markets, pending jurisdiction-specific price setting. The decreasing acceptable price ratio of iptacopan to C5 inhibitors alongside decreasing per-capita income spent on health care emphasizes the importance of jurisdiction-specific negotiations to yield value-informed pricing.71 In Brazil, a middle-income economy with noted persistent inequalities in health care utilization across socioeconomic strata, a distributional cost-effectiveness framework could be used to evaluate a value- and equity-informed evaluation of iptacopan monotherapy, should data across socioeconomic strata for patients living with PNH in Brazil become available.72 The narrow threshold ratio window between iptacopan being cost saving while increasing quality-adjusted life expectancy is because of drug cost accounting for a large portion of lifetime cost in the context of a modest quality-adjusted life expectancy increase with iptacopan in this patient population, narrowing further at lower WTP thresholds.
Our study has several limitations. First, the long-term effectiveness and safety of oral iptacopan monotherapy will not be known until sufficient postmarket authorization data are collected. Such data will be critical to inform the ongoing concern for intravascular hemolysis risk from missed doses and support the comparative efficacy of iptacopan as a frontline agent. It is conceivable that the benefit-risk profile of iptacopan could become less favorable with long-term use, thereby limiting its cost-effectiveness. Second, we did not include major adverse vascular events as a contributor to mortality, similarly because of uncertainty around iptacopan’s long-term safety data. However, because major adverse vascular events were not significantly different between the 2 trial arms in APPLY-PNH, this is not expected to affect our conclusion (ie, that iptacopan is cost saving). Third, we could not consider pegcetacoplan in this analysis to maintain modeling rigor; head-to-head data (whether randomized or same-patient cross-therapy comparison) would allow for this analysis. Finally, the utility values used in our base-case analysis were PNH specific but not directly derived from APPLY-PNH, which in the submission to NICE noted a differentially decreased quality of life for the same life states when on anti-C5 therapy (compared with iptacopan). To address this uncertainty, we conducted extensive sensitivity analyses and several scenario analyses, opting to not propagate a utility difference that would bias against the null hypothesis, showing all results consistent with the conclusion that iptacopan is the cost-effective strategy. In addition, we then separately applied APPLY-PNH trial-specific health state utilities reported to NICE, which expectedly showed even greater benefit with iptacopan.
Overall, with the successive approval of new therapeutics and the introduction of the Inflation Reduction Act, the United States is prioritizing value-based pricing, as many other countries have done for decades. As Medicare uses its new negotiating power, data that matches incremental clinical benefit to fair yet competitive pricing provides the groundwork for mutually beneficial drug development.73-75 Our study supports this framework within the expanding space of oral anticomplement drugs. We hope that our study will help support the use of iptacopan in anti-C5–treated patients living with PNH by directly addressing prior authorization concerns, all while decreasing health resource utilization, saving nursing and patient time, increasing patient transfusion independence, and ultimately, allowing for improved quality-adjusted life expectancy for patients living with PNH.
Acknowledgments
G.G. is supported by the NOMIS Foundation, Frederick A. DeLuca Foundation, Yale Bunker Endowment, Yale Cancer Center, and the National Institutes of Health, National Heart, Lung, and Blood Institute (grant 1K01 HL175220).
The contents of this article are solely the responsibility of the authors and do not necessarily represent the official views of the funding sources.
Authorship
Contribution: S.I., K.C., and G.G. conceived the study design; and all authors wrote and edited the manuscript.
Conflict-of-interest disclosure: H.M.K. has received options for Element Science and Identifeye and payments from F-Prime for advisory roles in the past 3 years; is a cofounder of, and holds equity in, Hugo Health, Refactor Health, and ENSIGHT-AI; and is associated with research contracts through Yale University from Janssen, Kenvue, Novartis, and Pfizer. A.C. has served as a consultant for MingSight, New York Blood Center, Pfizer, Sanofi, and Synergy; and has received authorship royalties from UpToDate. The remaining authors declare no competing financial interests.
Correspondence: George Goshua, Yale University School of Medicine, 333 Cedar St, New Haven, CT 06520; email: george.goshua@yale.edu.
References
Author notes
S.I. and K.C. are joint first authors.
Original data are available on request from the corresponding author, George Goshua (george.goshua@yale.edu).
The online version of this article contains a data supplement.
There is a Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.




Comments
Aligning cost-effectiveness analysis and cost-offset cap with affordable pricing
However, our main finding was clear: adopting iptacopan could lead to net cost savings compared to existing anti-C5 therapies, even at its current price. We believe highlighting these savings could incentivize payers and policymakers to facilitate patient access to iptacopan. Our intention is not to justify the current price but to objectively analyze the economic implications of adopting this innovation while recognizing the broader challenges around drug pricing.
Ultimately, we are fully aligned with the commenters' advocacy for affordable pricing, recognizing the ethical imperative to ensure that scientific advancements benefit all PNH patients. We encourage continued discussions and actions toward fairer drug pricing policies that match innovation with equitable patient access.
ARE WE, PNH SCIENTISTS, GUILTY?