In this issue of Blood, Ito et al1 present a compelling cost-effectiveness analysis of iptacopan for patients with paroxysmal nocturnal hemoglobinuria (PNH) who have suboptimal responses to the high-priced IV C5 inhibitors eculizumab2 and ravulizumab.3 Although C5 inhibitors were groundbreaking in their ability to target the terminal complement cascade and reduce intravascular hemolysis, they often left patients with persistent extravascular hemolysis and the burden of frequent infusions and transfusions. The advent of newer PNH therapies targeting earlier steps in the complement cascade, such as the subcutaneous C3 inhibitor pegcetacoplan4 and the oral factor B inhibitor iptacopan,5 has ushered in a new era of treatment possibilities, offering the potential for improved control of both intravascular and extravascular hemolysis. The study1 demonstrates that iptacopan not only improves outcomes for these patients but also yields long-term cost savings. This is a rare finding in independent cost-effectiveness analyses and challenges prior assessments questioning the economic value of iptacopan.6
Ito et al meticulously crafted a Markov model, a tool that simulates how patients might transition between different health states over time. The model's design considers which treatments are included, order in which they are given, duration of follow-up, location where treatment is provided, and clearly stated assumptions made to address data gaps or mirror real-world scenarios. Importantly, comparing their model with prior analyses demonstrates how the results of economic modeling can vary significantly depending on whose perspective is being considered: the patient, the insurer, the regional health system, or society. This is not a flaw of cost-effectiveness analysis but a vital feature, reflecting the complexities and trade-offs inherent to real-world health care decision-making.
Even for seasoned clinicians, rigorously developed economic models are shrouded in mystery, with results that are difficult to interpret and even harder to apply in practice. To translate cost-effectiveness data into action, the first step is to understand the key end points used by these models. One of the end points emphasized by Ito et al is the quality-adjusted life year (QALY), a measure that combines length and quality of life. Their QALY data offer valuable insights into how iptacopan promises to improve not just survival but also the lived experiences of patients who survive. Armed with this information, clinicians can engage in more meaningful shared decision-making with their patients, weighing the potential gains in quality of life against financial costs for both the individual patient and the health care system.
As a second step, clinicians must understand the clinical context of the findings and apply them with precision. The demonstrated cost-effectiveness of iptacopan is specific to patients with PNH who have not responded adequately to C5 inhibitors. Extrapolating these results to treatment-naïve patients based on this model, in practice or in guidelines, would be unjustified, because that would require a separate analysis. Moreover, it is important to note that key assumptions in this model differentiate it from a prior analysis that found iptacopan not to be cost-effective.6 In our view, 2 such choices that enhance the current model are recognizing that some patients still receive eculizumab even after the approval of ravulizumab and factoring in the quality-of-life benefits of oral medication over parenteral infusions. These considerations bring the model closer to actual clinical practice, in which patient preferences and treatment patterns can be complex.
As a third step, clinicians should keep in mind that a model is only as good as the quality of its underlying data. Randomized data, such as those from the APPLY-PNH5 trial that were used in this model, are best but not always available. For example, the lack of head-to-head trials comparing iptacopan with other promising treatments such as pegcetacoplan creates a knowledge gap that this analysis cannot address. This is partly due to the economic interests driving industry-sponsored trials and because the high cost of these therapies and rare nature of the disease limit independent or government-funded studies. There may be a role for combining model-based analyses with advanced statistical techniques such as network meta-analysis7 and matching-adjusted indirect comparison (MAIC)8 to bridge this evidence gap and inform treatment decisions in the absence of direct comparative data; however, applying these methods could also significantly increase the risk of biased results.
As a final step, clinicians must balance the societal or larger health system perspectives often taken in cost-effectiveness models with their duty to care for individual patients. One way for clinicians to do this is to actively advocate for access to cost-effective therapies within their practices’ pharmacy formularies and make informed treatment choices that consider both patient outcomes and the financial sustainability of the health care system. It is important to note that the favorable cost-effectiveness of iptacopan was found only in the context of the relatively high baseline cost of C5 inhibitors (eculizumab drug price >$40 000 per month, per Veterans Administration Federal Supply Schedule). Eculizumab, although highly effective,2 was not cost-effective compared with prior PNH therapies.9 Thus, this analysis should not be misconstrued as a justification for the exorbitant prices of either C5 inhibitors or iptacopan. These prices, precariously stacked like a house of cards, iptacopan atop ravulizumab atop eculizumab, must be challenged. Although clinicians have an ethical imperative to foster innovation for rare diseases, policymakers must implement measures to prevent price gouging and collusion.10 The introduction of iptacopan, pegcetacoplan, and, soon, danicopan into the PNH market, alongside the existing C5 inhibitors, offers hope for increased competition; however, cost containment will only be realized if payers and policymakers create an economic environment that addresses any misaligned incentives, such as those favoring in-office infusions over oral medications.
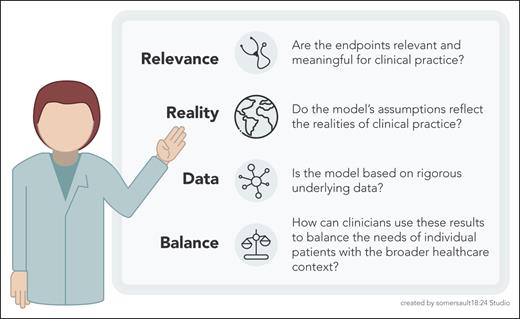
Ito et al have provided a robust economic analysis that also illuminates the complexities of cost-effectiveness research for rare diseases. Their findings are important for policymakers and payors and are also a call to action for clinicians who treat patients with PNH. Frontline hematologists must engage with these models, both to understand their assumptions and to participate actively in the ongoing dialogue about value in health care (see figure).
Cost-effectiveness checklist for clinicians. This figure outlines 4 key principles for clinicians to consider when evaluating and applying cost-effectiveness analyses in their practice. These principles emphasize the importance of using clinically relevant end points, realistic assumptions, rigorous data, and a balanced approach that considers both individual patient needs and the broader health care context.
Cost-effectiveness checklist for clinicians. This figure outlines 4 key principles for clinicians to consider when evaluating and applying cost-effectiveness analyses in their practice. These principles emphasize the importance of using clinically relevant end points, realistic assumptions, rigorous data, and a balanced approach that considers both individual patient needs and the broader health care context.
Conflict-of-interest disclosure: The authors declare no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal