In this issue of Blood, Banerjee et al1 present a secondary analysis of pooled data from 2 completed SWOG cooperative group trials (SO777 and S1211), showing that dose reductions in dexamethasone had no significant impact on postinduction progression-free survival (PFS) and overall survival (OS) in patients with newly diagnosed multiple myeloma.
Historically, with limited options for the treatment of multiple myeloma, dexamethasone was a necessary therapeutic partner to treat the disease.2,3 Dexamethasone doses used to invoke treatment responses in the past were as high as 40 mg for 4 days per week. Although dexamethasone demonstrates initial efficacy, especially at robust dosing, it also demonstrates significant and problematic toxicity over time. When the ECOG E4A03 study demonstrated increased mortality at this high 4-day pulse dexamethasone dosing (40 mg given 12 of 28 days) compared with 40 mg once weekly,4 we saw our first big decrease in dexamethasone dosing in myeloma treatment regimens. The recent exponential increase in the number of effective myeloma therapies and expanded use of quadruplet induction therapy brings into question the appropriate dose and duration of dexamethasone in contemporary myeloma treatment. In this exciting landscape of effective treatment options, it is unclear to what extent dexamethasone is necessary to treat disease. Dexamethasone is also used for symptom management and infusion reaction prophylaxis, although these supportive care indications generally do not require 40 mg.
With patients with multiple myeloma living longer than ever, it is important to minimize treatment toxicities. Providers and patients with myeloma are no strangers to the long list of bothersome steroid toxicities, including insomnia, heartburn, edema, psychiatric disturbances, muscle weakness, hyperglycemia, and diabetic complications. Too often, novel myeloma drugs are disrupted or abandoned due to symptoms that may, in fact, be dexamethasone induced. There is a need to better identify who may benefit from dexamethasone dose reduction. The practice of continuing full-dose dexamethasone despite significant steroid toxicities is likely a result of lacking data to guide dexamethasone-sparing strategies throughout the treatment course. National Comprehensive Cancer Network guidelines recommend limiting steroid use to the lowest possible effective dose in older adults5 but do not provide specific recommendations on how to determine that dose.
In this article, Banerjee et al add to the growing body of evidence that limiting dexamethasone exposure may be possible without adversely affecting treatment outcomes. This article builds on prior reports such as the ECOG E4A03 trial showing inferior OS with use of 4-day pulse dexamethasone regimens compared with 40 mg once weekly. Additionally, in a randomized phase 3 trial of lenalidomide plus continued dexamethasone vs lenalidomide plus fixed duration dexamethasone for 9 months, Larocca et al6 demonstrated reduced toxicity and an improved event-free survival and similar OS in older, intermediate-fit patients receiving less dexamethasone.6 Astonishingly, Banerjee et al found that less than a third of patients on stringently controlled clinical trials could tolerate full-dose dexamethasone on study. This finding of general dexamethasone intolerance makes it that much more important that their pooled analysis of SO777 and SO1211 trials also showed that dexamethasone dose reductions were not associated with decreased PFS or OS. These data highlight that individual patient characteristics, comorbidities, and experienced toxicities must be considered when dosing dexamethasone. In the setting of toxicity in which dose modification/reduction of a regimen is being considered, dexamethasone dosing has to be reevaluated as the potential etiology of the toxicity.
In conclusion, dexamethasone-sparing strategies can reduce steroid-induced toxicity and allow for patients to continue long-term myeloma treatment with improved quality of life. Many current studies limit the role of dexamethasone. Although the exact dosing and role of dexamethasone in contemporary treatment of multiple myeloma needs clearer definition, this study suggests that further reduction of this agent and its toxicity are possible (see figure). It is time to go down with dex!
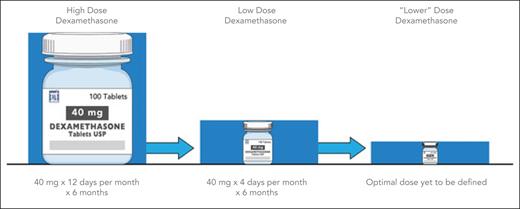
Historical trend toward optimal dexamethasone dosing. Review of dexamethasone dosing in the treatment of multiple myeloma shows a clear trend toward decreasing doses, with a need to determine the optimal dosing strategy.
Historical trend toward optimal dexamethasone dosing. Review of dexamethasone dosing in the treatment of multiple myeloma shows a clear trend toward decreasing doses, with a need to determine the optimal dosing strategy.
Conflict-of-interest disclosure: The authors declare no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal