Issue Archive
Table of Contents
BLOOD COMMENTARIES
BLOOD SPOTLIGHT
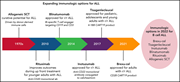
Approval of brexucabtagene autoleucel for adults with relapsed and refractory acute lymphocytic leukemia
Brexucabtagene autoleucel is the first anti-CD19 chimeric antigen receptor T (CAR-T) product approved for the treatment of relapsed/refractory acute lymphoblastic leukemia in adults. In this Blood Spotlight, Noelle Frey reviews the clinical trial results leading to approval, with a discussion of efficacy, toxicity, and the role of postremission hematopoietic stem cell transplantation.
CLINICAL TRIALS AND OBSERVATIONS
Long-term follow-up for the development of subsequent malignancies in patients treated with genetically modified IECs
Clinical Trials & Observations
Steffin and colleagues report on the development of secondary malignancies in 340 patients treated with genetically modified immune effector cells (IECs) from 27 different clinical trials over more than 1000 patient-years of follow-up. Secondary malignancy was seen in 16 patients (3.6%), comparable to what is seen with standard chemotherapy alone. Reassuringly, in 11 available biopsies, none carried the transgene, suggesting that IECs modified with gammaretroviral vectors do not increase the risk of secondary malignancy.
IMMUNOBIOLOGY AND IMMUNOTHERAPY
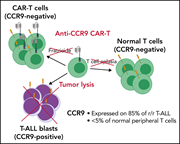
Anti-CCR9 chimeric antigen receptor T cells for T-cell acute lymphoblastic leukemia
Three article in this issue present preclinical data aimed at improving immunotherapeutic approaches for relapsed B- and T-cell acute lymphoblastic leukemia (T-ALL). Chimeric antigen receptor T (CAR-T) cell therapy directed against relapsed T-ALL is hampered by difficulty in identifying a target antigen that is not also expressed on healthy T cells, risking “fratricide” with T-cell aplasia and loss of the CAR-T cells. Maciocia and colleagues identify CCR9 as an antigen expressed on >85% of relapsed T-ALL and on <5% of normal T cells, demonstrating in cell lines and patient-derived xenografts that CAR-T cells targeting CCR9 have potent anti-leukemic activity without any evidence of fratricide. In the second article, Müller et al explore the potential of dual antibody targeting of CD47 and CD38 in a preclinical models of T-ALL; although CD47 does not avoid the risk of aplasia, it shows efficacy in combination with daratumumab in treating relapsed T-ALL and eradicating MRD. Finally, the third article addresses relapse following CD19-directed immunotherapies for B-ALL, which may be mediated by recurrence of CD19-negative leukemia. Bueno et al identify CD34+CD19−CD22+ cells in patients at diagnosis and relapse; they further show that CD22 expression precedes CD19 expression and that this population harbors the same genetic abnormalities of the B-ALL clone. They propose that this may represent a progenitor population that can give rise to B-ALL recurrence, supporting the use of combined CAR-T therapies, perhaps directed at both CD19 and CD22.
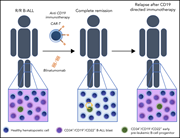
CD34+CD19−CD22+ B-cell progenitors may underlie phenotypic escape in patients treated with CD19-directed therapies
Brief Report
Three article in this issue present preclinical data aimed at improving immunotherapeutic approaches for relapsed B- and T-cell acute lymphoblastic leukemia (T-ALL). Chimeric antigen receptor T (CAR-T) cell therapy directed against relapsed T-ALL is hampered by difficulty in identifying a target antigen that is not also expressed on healthy T cells, risking “fratricide” with T-cell aplasia and loss of the CAR-T cells. Maciocia and colleagues identify CCR9 as an antigen expressed on >85% of relapsed T-ALL and on <5% of normal T cells, demonstrating in cell lines and patient-derived xenografts that CAR-T cells targeting CCR9 have potent anti-leukemic activity without any evidence of fratricide. In the second article, Müller et al explore the potential of dual antibody targeting of CD47 and CD38 in a preclinical models of T-ALL; although CD47 does not avoid the risk of aplasia, it shows efficacy in combination with daratumumab in treating relapsed T-ALL and eradicating MRD. Finally, the third article addresses relapse following CD19-directed immunotherapies for B-ALL, which may be mediated by recurrence of CD19-negative leukemia. Bueno et al identify CD34+CD19−CD22+ cells in patients at diagnosis and relapse; they further show that CD22 expression precedes CD19 expression and that this population harbors the same genetic abnormalities of the B-ALL clone. They propose that this may represent a progenitor population that can give rise to B-ALL recurrence, supporting the use of combined CAR-T therapies, perhaps directed at both CD19 and CD22.
LYMPHOID NEOPLASIA
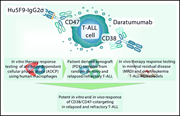
Combining daratumumab with CD47 blockade prolongs survival in preclinical models of pediatric T-ALL
Three article in this issue present preclinical data aimed at improving immunotherapeutic approaches for relapsed B- and T-cell acute lymphoblastic leukemia (T-ALL). Chimeric antigen receptor T (CAR-T) cell therapy directed against relapsed T-ALL is hampered by difficulty in identifying a target antigen that is not also expressed on healthy T cells, risking “fratricide” with T-cell aplasia and loss of the CAR-T cells. Maciocia and colleagues identify CCR9 as an antigen expressed on >85% of relapsed T-ALL and on <5% of normal T cells, demonstrating in cell lines and patient-derived xenografts that CAR-T cells targeting CCR9 have potent anti-leukemic activity without any evidence of fratricide. In the second article, Müller et al explore the potential of dual antibody targeting of CD47 and CD38 in a preclinical models of T-ALL; although CD47 does not avoid the risk of aplasia, it shows efficacy in combination with daratumumab in treating relapsed T-ALL and eradicating MRD. Finally, the third article addresses relapse following CD19-directed immunotherapies for B-ALL, which may be mediated by recurrence of CD19-negative leukemia. Bueno et al identify CD34+CD19−CD22+ cells in patients at diagnosis and relapse; they further show that CD22 expression precedes CD19 expression and that this population harbors the same genetic abnormalities of the B-ALL clone. They propose that this may represent a progenitor population that can give rise to B-ALL recurrence, supporting the use of combined CAR-T therapies, perhaps directed at both CD19 and CD22.
MYELOID NEOPLASIA
TP53 copy number and protein expression inform mutation status across risk categories in acute myeloid leukemia
Mutant TP53 is a well-recognized adverse risk factor in acute myeloid leukemia (AML), but the genotype-phenotype correlations of the heterogeneous TP53 mutations are poorly understood. Tashakori and colleagues performed an integrated genomic-proteomic analysis of 528 patients with AML, analyzing TP53 mutational status, copy number, and protein expression data that can begin to better risk-stratify this patient population to improve therapeutic decision-making.
LETTER TO BLOOD
Monoclonal and oligoclonal anti-platelet factor 4 antibodies mediate VITT
Kanack and colleagues analyze anti-platelet factor 4 antibodies from 5 patients with vaccine-induced thrombotic thrombocytopenia (VITT) secondary to COVID-19 adenoviral vaccination and antibodies from patients with spontaneous heparin-induced thrombocytopenia (HIT) and classical HIT. VITT antibodies are monoclonal or oligoclonal, similar to spontaneous HIT, whereas classical HIT antibodies are polyclonal. Heparin inhibits antibody-induced platelet activation in VITT, suggesting that heparin should be considered for the treatment of VITT.
BLOOD WORK
-
Cover Image
Cover Image
![issue cover]()
Mutant p53 protein accumulation (brown) in acute myeloid leukemia, depicted by immunohistochemistry in a bone marrow biopsy section. Mutant signals like this one, p53high, or p53truncated (not shown) correlate with and complement TP53 gene mutation and copy number status. An analysis of TP53 mutations, copy number status, and protein expression patterns in acute myeloid leukemia is presented in the article by Tashakori et al on page 58.
- PDF Icon Front MatterFront Matter
- PDF Icon Table of ContentsTable of Contents
- PDF Icon Editorial BoardEditorial Board
Advertisement intended for health care professionals
Email alerts
Advertisement intended for health care professionals





Safety of genetically modified T cells
Clinical Trials & Observations