In this issue of Blood, Bueno et al1 identify CD34+CD19negCD22+ leukemic progenitor populations and imply that these subclones may enable immune escape. These are critical findings, and they imply that there are and provide a potential mechanism for limitations on single-antigen targeting strategies and limitations on how we approach multiantigen targeting, particularly as the emergence of CD19neg B-cell acute lymphoblastic leukemia (B-ALL) is a primary mechanism of relapse after single-antigen immunotherapeutic targeting of CD19.2
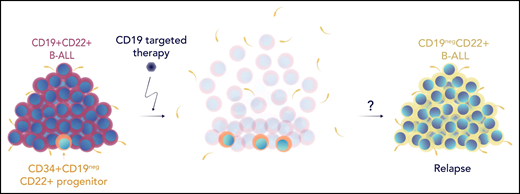
Bueno et al hypothesized that preexisting CD19neg populations could serve as a reservoir of cells that could emerge after clearance of bulk CD19+ disease, so they first sought to characterize the expression of CD22 across the B-cell developmental pathway. By studying the expression of CD34+ hematopoietic stem cell progenitor (HSCP) populations across healthy fetal, neonatal, and postnatal samples using bulk, single-cell RNA sequencing and flow cytometry, they demonstrate that the expression of CD22 precedes that of CD19. They further identify the existence of CD34+CD19negCD22+ populations in patients with B-ALL at both diagnosis and relapse (see figure). Although this population primarily represents a preleukemic population, the additional investigations of Bueno et al support a hypothesis that this population is more prevalent after CD19-directed therapy, can harbor oncogenic molecular lesions and, based on an in vivo model, can repopulate CD19neg leukemia. In addition, they found that patients who relapse after CD19-directed therapy trended toward having relatively higher fractions of the CD34+CD19negCD22+ population at diagnosis than patients who remained in remission. This supports the notion that this cell population may serve as a reservoir for subsequent relapse. However, because of the small sample size, further study in a larger cohort is needed to confirm these findings.
The figure shows leukemia cells lumped together in the form of a haystack, layered as if it is a developmental pathway with a single cell, showing CD34+CD19negCD22+ at the bottom.
The figure shows leukemia cells lumped together in the form of a haystack, layered as if it is a developmental pathway with a single cell, showing CD34+CD19negCD22+ at the bottom.
The notion of resistance to CD19-directed therapies stemming from HSCPs has previously been described in the context of BCR-ABL1-positive ALL, in which involvement of BCR-ABL in HSCPs resulted in development of BCR-ABL1-positive acute monoblastic leukemia after treatment with blinatumomab, a CD19-CD3 bispecific T-cell engager.3 Furthermore, the identification of low-level preexisting CD19neg populations has also been of concern.4 Studies that evaluated sequential CD19 targeting strategies (eg, blinatumomab before CD19 chimeric antigen receptor [CAR] T cells)5-7 raised additional concerns about the impact of continued CD19 targeting and concerns that evolving CD19 expression may predispose to CD19neg relapse. The Bueno et al study expands previous efforts and raises an additional consideration regarding limitations to single-antigen CD19 targeting.
In this regard, the phylogenetic appearance of CD22 before the appearance of CD19 described by Bueno et al is of particular interest, especially because highly active CD22 targeting therapies are available or in active development. Inotuzumab ozogamicin, a CD22-targeted antibody conjugate, is approved for adults with B-ALL, and pediatric studies support its efficacy in childhood B-ALL.8 CD22 CAR T cells have also emerged as a highly effective strategy.9 As with CD19 targeting, however, experience with both CD22-targeted strategies has been marred with CD22 modulation emerging as a mechanism of escape and with a predisposition to CD22– with sequential targeting.8,9 Thus, achieving long-term durable remission, the ultimate goal of these novel therapies, may require further fine-tuning in strategic approach and timing. Certainly, the development of effective CD22 targeting as a critically important target in B-ALL needs to be prioritized.
Thus, strategies toward combinatorial targeting and optimizing sequential approaches is not only scientifically sound but urgently needed to improve both depth and duration of remission. Given the critical importance of minimal residual disease in B-ALL, emerging data in support of lingering populations raises concerns about incomplete disease eradication. Using investigative tools to identify minuscule populations beyond those detected by flow cytometry will be imperative. In addition, the potential concurrent downregulation of CD22 that has been seen with CD19 loss6,10 and the appearance of CD22 earlier in HSCP development suggest that perhaps when sequential strategies are needed, targeting CD22 before targeting CD19 may be a rational approach and should be considered.
Standard-of-care approaches incorporating non–cross-resistant, multiagent chemotherapy evolved from limitations of single-agent chemotherapy, the latter of which was fraught with incomplete response, development of resistance, and rapid relapse. Single-antigen–targeted immunotherapy is likely to be no different, particularly for diseases in which the underlying proliferative rate may more rapidly lead to emergence of antigen-negative cases. The proverbial “needle in a haystack” underscores the critical need to identify preexisting antigen-negative populations, particularly as single-antigen–targeted therapies move to the forefront and tools to detect such populations are becoming more broadly available. Collectively, the work by Bueno et al adds an additional consideration by identifying potential reservoirs of antigen-negative populations that can emerge after CD19 targeting, which provides further support for the importance of sequential or concurrent CD22 targeting in B-ALL.
Conflict-of-interest disclosure: T.J.F. is an employee of Sana Biotechnology. N.N.S. declares no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal