Issue Archive
Table of Contents
EDITORIAL
Introduction to a How I Treat series on plasma cell dyscrasias
The treatment paradigms for multiple myeloma and amyloidosis have been transformed by novel therapies and continue to be modified in the face of new immunologic agents. In this How I Treat series on plasma cell dyscrasias edited by Associate Editor Hervé Avet-Loiseau, experts in the field discuss the rapidly changing treatment landscape for multiple myeloma and light-chain amyloidosis. Using illustrative cases, they review the role of autologous transplant, novel combination therapies, and the impact of new immunotherapy approaches to these complex and heterogeneous diseases.
BLOOD COMMENTARIES
PLENARY PAPER
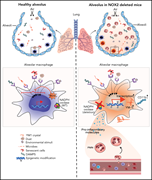
Macrophage NOX2 NADPH oxidase maintains alveolar homeostasis in mice
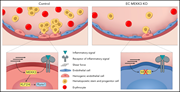
Chronic granulomatous disease (CGD) is caused by mutations of the leukocyte NADPH oxidase 2 (NOX2) and is associated with increased infections. It is also characterized by sterile inflammation, the mechanism of which is poorly understood. In this Plenary paper, Bhattacharya et al demonstrate in a mouse model of CGD that normal alveolar macrophages (AMs) have an anti-inflammatory influence on lung inflammation and that AMs arising from NOX2-null monocytes stimulate inflammatory pathways, suggesting an explanation for excess non-infectious inflammation.
PERSPECTIVE
Treatment and clinical endpoints in polycythemia vera: seeking the best obtainable version of the truth
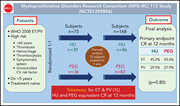
Therapy for essential thrombocythemia (ET) and polycythemia vera (PV) is aimed at reducing thrombotic risk through normalizing blood counts. Mascarenhas et al report on a randomized trial of hydroxyurea (HU) vs pegylated interferon α (PEG) in 168 patients with ET and PV who are at a high risk for vascular complications. They found that there is no significant difference between the 2 agents in CR rates at 12 months or in the rates of thrombotic events and disease progression. In an accompanying Perspective, Jason Gotlib places these results in the context of other studies, emphasizing the place of standard aspirin and phlebotomy, and speculating on the best use of HU, PEG, ruxolitinib, and novel agents in the treatment of ET and PV, while outlining gaps in our understanding that limit the certainty of management decisions.
HOW I TREAT SERIES
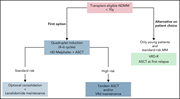
How I treat frontline transplantation-eligible multiple myeloma
Clinical Trials & Observations
The treatment paradigms for multiple myeloma and amyloidosis have been transformed by novel therapies and continue to be modified in the face of new immunologic agents. In this How I Treat series on plasma cell dyscrasias edited by Associate Editor Hervé Avet-Loiseau, experts in the field discuss the rapidly changing treatment landscape for multiple myeloma and light-chain amyloidosis. Using illustrative cases, they review the role of autologous transplant, novel combination therapies, and the impact of new immunotherapy approaches to these complex and heterogeneous diseases.
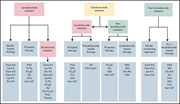
How I treat high-risk multiple myeloma
The treatment paradigms for multiple myeloma and amyloidosis have been transformed by novel therapies and continue to be modified in the face of new immunologic agents. In this How I Treat series on plasma cell dyscrasias edited by Associate Editor Hervé Avet-Loiseau, experts in the field discuss the rapidly changing treatment landscape for multiple myeloma and light-chain amyloidosis. Using illustrative cases, they review the role of autologous transplant, novel combination therapies, and the impact of new immunotherapy approaches to these complex and heterogeneous diseases.
How I treat relapsed multiple myeloma
Clinical Trials & Observations
The treatment paradigms for multiple myeloma and amyloidosis have been transformed by novel therapies and continue to be modified in the face of new immunologic agents. In this How I Treat series on plasma cell dyscrasias edited by Associate Editor Hervé Avet-Loiseau, experts in the field discuss the rapidly changing treatment landscape for multiple myeloma and light-chain amyloidosis. Using illustrative cases, they review the role of autologous transplant, novel combination therapies, and the impact of new immunotherapy approaches to these complex and heterogeneous diseases.
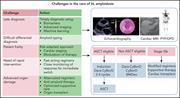
How I treat AL amyloidosis
The treatment paradigms for multiple myeloma and amyloidosis have been transformed by novel therapies and continue to be modified in the face of new immunologic agents. In this How I Treat series on plasma cell dyscrasias edited by Associate Editor Hervé Avet-Loiseau, experts in the field discuss the rapidly changing treatment landscape for multiple myeloma and light-chain amyloidosis. Using illustrative cases, they review the role of autologous transplant, novel combination therapies, and the impact of new immunotherapy approaches to these complex and heterogeneous diseases.
CLINICAL TRIALS AND OBSERVATIONS
A randomized phase 3 trial of interferon-α vs hydroxyurea in polycythemia vera and essential thrombocythemia
Clinical Trials & Observations
Therapy for essential thrombocythemia (ET) and polycythemia vera (PV) is aimed at reducing thrombotic risk through normalizing blood counts. Mascarenhas et al report on a randomized trial of hydroxyurea (HU) vs pegylated interferon α (PEG) in 168 patients with ET and PV who are at a high risk for vascular complications. They found that there is no significant difference between the 2 agents in CR rates at 12 months or in the rates of thrombotic events and disease progression. In an accompanying Perspective, Jason Gotlib places these results in the context of other studies, emphasizing the place of standard aspirin and phlebotomy, and speculating on the best use of HU, PEG, ruxolitinib, and novel agents in the treatment of ET and PV, while outlining gaps in our understanding that limit the certainty of management decisions.
HEMATOPOIESIS AND STEM CELLS
Endothelial MEKK3-KLF2/4 signaling integrates inflammatory and hemodynamic signals during definitive hematopoiesis
PLATELETS AND THROMBOPOIESIS
Inhibition of LDHA to induce eEF2 release enhances thrombocytopoiesis
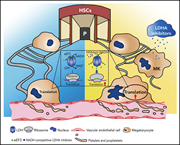
Chen et al describe a surprising role for lactate dehydrogenase A (LDHA) in regulating translation of platelet-specific proteins that influence megakaryocyte maturation and platelet number. This pathway may offer a novel target for the treatment of thrombocytopenia.
THROMBOSIS AND HEMOSTASIS
TRANSPLANTATION
BET-bromodomain and EZH2 inhibitor–treated chronic GVHD mice have blunted germinal centers with distinct transcriptomes
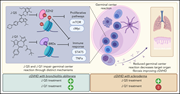
Sclerodermatous and pulmonary chronic graft-versus-host disease (cGVHD) is highly resistant to therapy. Previous studies suggest these fibrotic syndromes are germinal center dependent. Zaiken and colleages report that blocking germinal center development with BET bromodomain and EZH2 inhibitors improves these cGVHD syndromes in a murine model, setting the stage for clinical trials of these agents for the treatment of human cGVHD.
BLOOD WORK
-
Cover Image
Cover Image
![issue cover]()
Immunofluorescence image of a splenic germinal center from a mouse with chronic graft-versus-host disease (cGVHD). The section was stained with peanut agglutinin–rhodamine, DAPI (4′-6-diamidino-2-phenylindole), and CD4–fluorescein isothiocyanate. The central red structure shows the germinal center with T-cell (green) areas flanking it. Germinal centers are enlarged in mice with cGVHD and were effectively reduced in both size and frequency with treatment by a novel EZH2 inhibitor, JQ5, and by a BET-bromodomain inhibitor, JQ1. See the article by Zaiken et al on page 2983.
- PDF Icon Front MatterFront Matter
- PDF Icon Table of ContentsTable of Contents
- PDF Icon Back MatterBack Matter
- PDF Icon Editorial BoardEditorial Board
Advertisement intended for health care professionals
Email alerts
Advertisement intended for health care professionals





NOX2: is the best defense a good offense?