Issue Archive
Table of Contents
BLOOD COMMENTARIES
HOW I TREAT
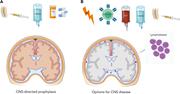
How I prevent and treat central nervous system disease in adults with acute lymphoblastic leukemia
Clinical Trials & Observations
Systemic treatment of adult patients with acute lymphoblastic leukemia continues to evolve with the introduction of targeted and T-cell activating therapies, but central nervous system (CNS) disease remains a challenge. In this context, Kopmar and Cassaday illustrate their approaches to prevention and treatment of CNS leukemia with 4 clinical vignettes, summarizing current knowledge and laying out their preferred strategies in instances where high-quality evidence is lacking.
CLINICAL TRIALS AND OBSERVATIONS
A phase 2 study of interleukin-22 and systemic corticosteroids as initial treatment for acute GVHD of the lower GI tract
Clinical Trials & Observations
Acute lower gastrointestinal (GI) graft-versus-host disease (aGVHD) remains a leading cause of mortality and morbidity postallograft. Ponce et al report on the results of a multicenter single-arm, phase 2 study evaluating the safety and efficacy of a novel recombinant human interleukin-22 dimer in combination with systemic corticosteroids for newly diagnosed GI aGVHD. With no dose-limiting toxicity identified and a 70% day 28 response rate with associated expansion of healthy commensal GI flora, these data suggest acceptable safety and efficacy deserving of phase 3 evaluation.
Hydroxyurea treatment is associated with lower malaria incidence in children with sickle cell anemia in sub-Saharan Africa
Clinical Trials & Observations
The majority of children with sickle cell anemia (SCA) live in sub-Saharan Africa (SSA). Hydroxyurea remains the most effective and affordable option for disease-modifying therapy in resource-limited countries. In an open-label trial among children with SCA in 4 countries in SSA, Olupot-Olupot and colleagues report that hydroxyurea therapy at maximum-tolerated doses is associated with a lower malaria incidence, suggesting, but not proving, that there may be an additional benefit of this intervention in these circumstances.
IMMUNOBIOLOGY AND IMMUNOTHERAPY
LYMPHOID NEOPLASIA

Proposed integrative model of oncogenic TCL1A through the promotion of genomic instability in malignant B-cells. Overexpression of TCL1A causes genomic instability via multiple pathways: (i) deficient repair of DNA damage, (ii) an accelerated cell cycle transition, (iii) susceptibility to aneuploidy (data for i-iii presented in this report), and (iv) apoptosis resistance (published before). Key molecules through which these effects (i.e. via physical interaction with TCL1A) are mediated are highlighted. We show here that overexpression of TCL1A leads to a decreased phosphorylation of ATM and reduced expression of p53 upon genotoxic stress. This might cause impaired sensing and processing of DNA insults (exogeneous or as part of DNA replication), and deficient G2/M checkpoint control, contributing to (i) and (ii). The interaction of TCL1A with molecules from the mitotic checkpoint complex (MCC), including CDC20, might form the basis of the observed accelerated cell cycle transition by deregulating the mitotic checkpoint, contributing to (ii) and (iii). Furthermore, the high frequency of aberrant spindles seen in TCL1A-overexpressing cells promotes the observed aneuploidy. The already well-established role of TCL1A in augmenting AKT phosphorylation6,13,16 leads to increased survival signaling and thereby to apoptotic resistance (perturbation of a safeguarding mechanism). Together, TCL1A overexpression leads to a premature, DNA-damage-prone cell cycle checkpoint transition, which contributes to the TCL1A transforming capability in concert with impaired repair and hyperactive pro-survival signaling.
The proto-oncogene TCL1A deregulates cell cycle and genomic stability in CLL
Heightened expression of the proto-oncogene TCL1A is associated with aggressive biology in chronic lymphocytic leukemia (CLL). Using primary CLL cells and murine models, Stachelscheid and colleagues describe the pleiotropic effect of this proto-oncogene as a major factor in CLL pathogenesis through acceleration of premature and DNA damage-prone cell-cycle transition, causing aneuploidy, impaired DNA damage repair, increased cell-survival signaling, as well as apoptotic resistance. These data provide novel insights requiring further investigation.
MYELOID NEOPLASIA
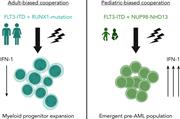
FLT3ITD drives context-specific changes in cell identity and variable interferon dependence during AML initiation
Leukemic driver gene alterations that co-occur with FLT3 internal tandem duplication (FLT3ITD) in acute myeloid leukemia (AML) vary with age; chromosomal rearrangements are more common in children and single-gene mutations, such as DNMT3A, and RUNX1 loss of function mutations are more common in adult AML. Li and colleagues explore the significance of those observations using murine models with complementary transcriptomic, epigenetic, and functional studies. The authors reveal that the functional consequences of FLT3ITD signaling are dynamic, demonstrating age-, cell-type–, and comutation-specific dependencies, which help explain divergent clinical phenotypes.
THROMBOSIS AND HEMOSTASIS
A nanobody against the VWF A3 domain detects ADAMTS13-induced proteolysis in congenital and acquired VWD
Removal of the vicinal disulfide enhances the platelet-capturing function of von Willebrand factor
Brief Report
A balance between intramolecular interactions and hemodynamic forces keeps the flexible multimeric protein von Willebrand factor (VWF) from binding circulating platelets while in the plasma. Upon immobilization, VWF unravels and elongates in response to fluid flow, activating specific monomers within the multimer to drive platelet adhesion. Using functional studies of wild type and mutated recombinant proteins, Tischer et al refine our understanding of how allostery contributes to these processes.
TRANSPLANTATION
The OTUD1-Notch2-ICD axis orchestrates allogeneic T cell–mediated graft-versus-host disease
Notch signaling is critical to immune cell development and regulates mature T-cell activation, differentiation, and function during acute graft-versus-host disease (aGVHD). Previous attempts to directly target Notch signaling for aGVHD therapy have shown efficacy but also problematic toxicity. Taking a less direct path, Cheng and colleagues identify that targeting ovarian tumor deubiquitinase 1 (OTUD1) leads to inhibition of Notch2 signaling and abrogation of aGVHD in murine models, potentially reflecting a novel strategy for clinical exploration.
LETTER TO BLOOD
Safety and efficacy of CPX-351 in younger patients (<60 years old) with secondary acute myeloid leukemia
Clinical Trials & Observations
BLOOD WORK
ERRATA
-
Cover Image
Cover Image
![issue cover]()
Hematoxylin- and eosin-stained lung sections from mice with acute graft-versus-host disease (aGVHD) transferred with OTUD1-deficient bone marrow cells and splenocytes and over-expressed Notch2-ICD (NICD). NICD aggravates the severity of aGVHD in mice transferred with OTUD1-deficient donor cells. See the article by Cheng et al on page 1474.
- PDF Icon Front MatterFront Matter
- PDF Icon Table of ContentsTable of Contents
- PDF Icon Back MatterBack Matter
- PDF Icon Editorial BoardEditorial Board
Advertisement intended for health care professionals
Email alerts
Advertisement intended for health care professionals







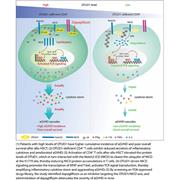

IL-22, a new beacon in gastrointestinal aGVHD
Clinical Trials & Observations