Issue Archive
Table of Contents
BLOOD COMMENTARIES
CLINICAL TRIALS AND OBSERVATIONS
Phase 1/2 study of uproleselan added to chemotherapy in patients with relapsed or refractory acute myeloid leukemia
Clinical Trials & Observations
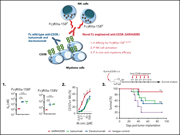
Building on compelling preclinical research, DeAngelo et al report the results of a phase 1/2 trial of uproleselan, an E-selectin inhibitor, in combination with 2 different standard chemotherapy regimens in patients with acute myeloid leukemia. Their data suggest antileukemic activity through targeting of the bone marrow microenvironment and form the basis for a randomized trial.
Phase 1 study of oral azacitidine (CC-486) plus R-CHOP in previously untreated intermediate- to high-risk DLBCL
Clinical Trials & Observations
Martin and colleagues report the results of a phase 1b trial of adding oral azacitidine (CC-486) to R-CHOP. “Priming” with CC-486 is shown to cause extensive genomewide hypomethylation in paired tumor biopsies, and the combination demonstrates promising clinical activity in patients with previously untreated intermediate- to high-risk aggressive B-cell lymphomas. This concept is being further explored in a phase 2/3 randomized study of R-miniCHOP with or without CC-486 in older patients.
R-CHOP in DLBCL: priming for success
Clinical Trials & Observations
IMMUNOBIOLOGY AND IMMUNOTHERAPY
SAR442085, a novel anti-CD38 antibody with enhanced antitumor activity against multiple myeloma
Systemic IL-15 promotes allogeneic cell rejection in patients treated with natural killer cell adoptive therapy
Clinical Trials & Observations
Brief Report
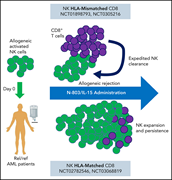
Natural killer (NK) cells are a promising alternative to T cells for allogeneic cellular immunotherapy, but the optimal cytokine support after adoptive transfer to promote NK cell expansion, persistence, and antileukemic effect remains unclear. Through indirect comparisons with systemic interleukin-2 (IL-2) across two trials, Berrien-Elliott et al report that N-803, an IL-15 receptor agonist, induces a higher early peak of donor NK cell expansion, but less persistence and reduced activity against acute myeloid leukemia, due to allogeneic cell rejection by host T cells. This unexpected result provides a cautionary lesson for future trials in this field.
LYMPHOID NEOPLASIA
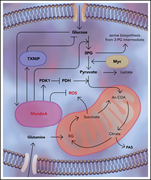
MondoA drives malignancy in B-ALL through enhanced adaptation to metabolic stress
Clonal hematopoiesis, myeloid disorders and BAX-mutated myelopoiesis in patients receiving venetoclax for CLL
Clinical Trials & Observations
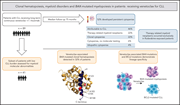
Blombery et al report on the clonal dynamics of myelopoiesis in patients with relapsed chronic lymphocytic leukemia who received the BCL2 inhibitor venetoclax after DNA-damaging prior chemotherapy. They identify emergence of late-onset clonal hematopoiesis and lossof-function mutations in the apoptotic activator BAX in myeloid clones. This study shows that a targeted therapy can select for resistant mutations not only in malignant populations but also within normal tissue displaying clonal mosaicism.
MYELOID NEOPLASIA
Identification and prioritization of myeloid malignancy germline variants in a large cohort of adult patients with AML
Clinical Trials & Observations
It is increasingly apparent that acute myeloid leukemia (AML) germline predisposition mutations are recognizable by tumor sequencing. Yang and colleagues present matched tumor germline analyses for 391 AML patients from the Beat AML cohort, finding that 13.6% of patients unselected by family history have pathogenic or likely pathogenic variants, with DDX41 and DNA damage response pathway genes most frequently represented. These data highlight the need to detect and respond to such findings and portend greater attention to the affected genes in studies of AML pathogenesis.
PHAGOCYTES, GRANULOCYTES, AND MYELOPOIESIS
Resolvin T-series reduce neutrophil extracellular traps
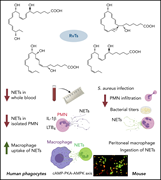
Resolution of the acute inflammatory response is now widely recognized as an active process that includes biosynthesis of a superfamily of endogenous lipid mediators, including resolvins. Chiang et al combine studies of human neutrophils with animal disease models to reveal the role of 13-series (T-series) resolvins in reducing neutrophil extracellular net (NET) formation, particularly facilitating macrophage clearance of NETs in the context of bacterial and viral infections. This work identifies a novel mechanism for the resolution of infections and associated coagulopathies.
THROMBOSIS AND HEMOSTASIS
PKM2 promotes neutrophil activation and cerebral thromboinflammation: therapeutic implications for ischemic stroke
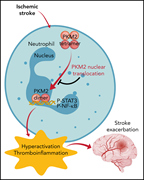
Neutrophils are key effector cells in the pathogenesis of stroke. Using in vivo murine models, Dhanesha and colleagues identify the multifunctional protein pyruvate kinase muscle 2 (PKM2) in neutrophils as a key modulator of outcome. Induction of stroke results in nuclear translocation of PKM2 in neutrophils driving a thromboinflammatory reaction through STAT3 signaling that exacerbates the severity of cerebral ischemia-reperfusion injuries, a process that is potentially amenable to timely, targeted pharmacological inhibition.
LETTERS TO BLOOD
Clinical, pathological, and molecular features of myelodysplasia cutis
Clinical Trials & Observations
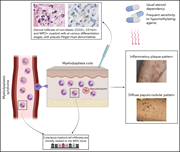
Applying next-generation sequencing to paired skin and marrow biopsies, Delaleu et al describe an entity they term “myelodysplasia cutis,” comprising histologic features of histiocytoid Sweet syndrome in the skin and a clonally related myelodysplasia in the bone marrow. This can be distinguished from forms of histiocytoid Sweet syndrome not associated with myelodysplastic syndromes, as well as classical neutrophilic Sweet syndrome associated with various malignant and nonmalignant diseases and leukemia cutis where the infiltrating cells are myeloblasts.
BLOOD WORK
ERRATA
-
Cover Image
Cover Image
![issue cover]()
Myeloperoxidase-expressing (brown) myeloid inflammatory infiltrate consisting of metamyelocytes or band neutrophils, some with pseudo–Pelger-Huët abnormality, in the setting of myelodysplasia cutis. See the article by Delaleu et al on page 1251.
- PDF Icon Front MatterFront Matter
- PDF Icon Table of ContentsTable of Contents
- PDF Icon Back MatterBack Matter
- PDF Icon Editorial BoardEditorial Board
Advertisement intended for health care professionals
Email alerts
Advertisement intended for health care professionals






A nonstick marrow may help to fry leukemia
Clinical Trials & Observations