In this issue of Blood, Martin et al1 report the results of a phase 1 study evaluating a novel strategy of epigenetic priming before rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone (R-CHOP) using the hypomethylating agent CC486, an oral azacitidine, as initial treatment in patients with high-risk diffuse large B-cell lymphoma (DLBCL) or grade 3B/transformed follicular lymphoma. The authors demonstrate that the addition of CC-486 to R-CHOP shows promising clinical antitumor activity with an acceptable safety profile.
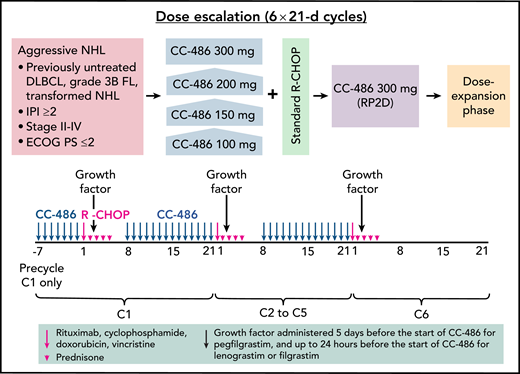
In this phase 1 study, CC-486 was administered once daily as a 7-day priming regimen before the first cycle of R-CHOP. Subsequently, CC-486 was given daily for 14 days (days 8-21) during cycles 1 to 5 of R-CHOP, with none administered during cycle 6 (see figure). The study examined 4 escalating dose levels of CC-486, specifically 100, 150, 200, and 300 mg daily. For efficient determination of the maximum tolerated dose (MTD), the time-to-event continual reassessment method was used to assign dose levels in a staggered manner without the need to pause for complete follow-up of already enrolled patients. Although the MTD was not reached in this study, the recommended phase 2 dose was 300 mg, supported by the identical approved single-agent dose in adult patients with acute myeloid leukemia in remission.2
CC‐486 DLBCL‐001 study design and dosing schedule. ECOG PS, Eastern Cooperative Oncology Group performance status; FL, follicular lymphoma; IPI, International Prognostic Index; NHL, non-Hodgkin lymphoma; RP2D, recommended phase 2 dose. See Figure 1 in the article by Martin et al that begins on page 1147.
CC‐486 DLBCL‐001 study design and dosing schedule. ECOG PS, Eastern Cooperative Oncology Group performance status; FL, follicular lymphoma; IPI, International Prognostic Index; NHL, non-Hodgkin lymphoma; RP2D, recommended phase 2 dose. See Figure 1 in the article by Martin et al that begins on page 1147.
Most patients had high-risk disease, with 76.3% age ≥60 years, 59.3% with an IPI score ≥3, and 93.2% with advanced stage III/IV disease. Among the 59 evaluable patients, the complete response (CR) and overall response (OR) rates were 88.1% and 94.9%, respectively, and for the 40 patients in the 300-mg dose-escalation/expansion cohort, CR and OR rates were 87.5% and 95.0%, respectively. These rates compare favorably with those reported in other combination trials of R-CHOP plus drug X, including the phase 2 CAVALLI study3 (incorporating venetoclax) with CR and OR rates of 69% and 83%, the ROBUST study4 (adding lenalidomide) with rates of 69% and 91%, and the PHOENIX study5 (using ibrutinib) with rates of 67% and 89%, respectively.
Notwithstanding the fact that this was a phase 1 dose-finding study with limited patient numbers, the 2-year progression-free survival (PFS) rates were encouraging at 78.6% and 72.4% for the entire population and the 300-mg dose-escalation/expansion cohort, respectively. Again, these rates are similar to those seen with other R-CHOP combination trials, including 78% to 80% in CAVALLI (venetoclax),3 75% in ROBUST (lenalidomide),4 70% in PHOENIX (ibrutinib),5 and 74.3% (30-month PFS) in REMoDL-B (bortezomib).6
Toxicities were manageable and were consistent with each of the known safety profiles of CC-486 and R-CHOP. The most common treatment-emergent adverse events were gastrointestinal, with an incidence of all-grade nausea of 57.6%, and hematologic, with rates of grade 3/4 neutropenia of 62.7% and febrile neutropenia of 25.4%. Similar hematologic toxicities were seen with venetoclax plus R-CHOP, with rates of grade 3/4 neutropenia and febrile neutropenia of 68% and 23%, respectively. These and other studies confirm the need for clear, prophylactic strategies when novel agents are added to the R-CHOP backbone.
Epigenetic dysregulation is a substantial contributor to the pathogenesis of DLBCL. Epigenetically inhibiting DNA/histone modifications such as methylation and acetylation has been shown to induce chemosensitization in DLBCL.7 Hypomethylating agents such as azacitidine, although not directly causing prominent cytotoxicity, can lead to priming of lymphoma cells by inducing a newly acquired vulnerability to R-CHOP chemotherapy. In support of the mechanism of epigenetic priming by CC-486, the authors undertook several correlative studies and demonstrated upregulated expression of hypermethylated CpG islands associated with specific transposable elements, as well as upregulation of interferon family molecules, which together may contribute to antitumor immunity.
When combining a novel agent with R-CHOP, dose intensity of each can be compromised. In this study, both absolute dose intensity (ADI) and relative dose intensity (RDI) were favorable, although mildly reduced. The mean overall RDI of CC-486 plus R-CHOP was 89.6%, with 42 patients (69.5%) achieving an RDI >85%, whereas the mean RDI of the 300-mg dose of CC-486 was 82.2%; for R-CHOP, it was 96.2%. Certainly, this trial and other combination studies highlight the need for maintaining high levels of ADI and RDI of both the novel agent and the chemotherapy backbone, particularly when treating older high-risk patients.
Diagnosis-to-treatment interval (DTI) has emerged as a prognostic factor in DLBCL. By reflecting tumor burden and clinical urgency, shorter DTIs are associated with inferior survival outcomes.8 In the current study, the median DTI was 28 days, suggesting that many enrolled patients may not have required urgent treatment. For future trials in DLBCL, DTI may become more relevant as trials seek to stratify for high-risk baseline genetic features such as double-hit gene rearrangements and molecular signatures, as well as poor-risk DLBCL molecular subtypes or genetic clusters, whereby timely assay completion may represent a logistic challenge. As efforts are being made to overcome these potential barriers, ongoing clinical trials will still need to use conventional prognostic scores for patient enrichment, namely IPI score of 3 to 5 for those age >60 years and age-adjusted IPI score of 2 to 3 for those age ≤60 years.9
Older patients with DLBCL age >75 years represent a particular challenge. These patients often exhibit impaired performance scores, functional capacities, and multimodal scores, as well as low albumin levels, each of which is associated with greater toxicity and inferior outcomes.10 In quantitative terms, impaired delivery of full-dose R-CHOP chemotherapy in older patients manifests as reduced levels of ADI and RDI because of dose delays, dose reductions, and drug omissions. In the upcoming phase 2/3 trial, by combining reduced-dose R-CHOP with CC-486 in an older DLBCL cohort, it is postulated that the planned dose intensities can be maintained over the entire treatment course and finally yield more encouraging results for this challenging group of patients.
Conflict-of-interest disclosure: M.H. has received honoraria or served as an advisor for Takeda, Roche, Janssen, Beigene, Merck Sharp & Dohme, and Gilead.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal