In this issue of Blood, Berrien-Elliott et al1 report decreased clinical activity of haploidentical natural killer (NK) cell therapy using IL-15/N-803 support due to accelerated host T-cell–mediated rejection, which limits NK cell persistence.
NK cells represent a promising cell type in antitumor therapy based on both efficacy and safety. NK cell-based adoptive immunotherapy of patients with hematological malignancies is an emerging field in clinical trials.2 NK cell protocols have been based on the infusion of purified allogeneic NK cells, either resting or activated in vitro with cytokines (eg, interleukin [IL]-2 or IL-15). However, in vivo NK cell expansion and persistence require support by cytokines that are administered either intravenously or subcutaneously. In the pioneering study by Miller et al,3 after a cyclophosphamide/fludarabine (Cy/Flu) preparative regimen, adult patients with acute myeloid leukemia (AML) received infusion of IL-2–activated NK cells derived from haploidentical donors (ie, a relative sharing only one HLA-haplotype with the patient) followed by subcutaneous injection of IL-2. Higher dose Cy/Flu was associated with higher serum IL-15 concentrations and more prolonged in vivo NK cell detection. To prevent the IL-2–mediated stimulation of host regulatory T cells (Treg),4 other clinical trials explored the alternative use of rhIL-15.5 These phase I/II studies showed improved NK cell expansion in vivo but also higher incidence of side effects such as cytokine release syndrome and neurotoxicity after rhIL-15 given subcutaneously but not intravenously.5 To increase the in vivo half-life of IL-15, the IL-15 superagonist N-803 (formerly known as ALT-803) was developed and tested in patients with hematologic malignancies who had relapsed after allogeneic hematopoietic stem cell transplantation (HSCT).6 This phase I study documented the safety of N-803 given subcutaneously. Furthermore, the sustained serum cytokine levels stimulated activation and expansion of NK and CD8+ T cells without affecting Treg. In recent years, the use of IL-15, IL-12, and IL-18 preactivation before adoptive transfer has been proposed to induce the differentiation of memory-like (ML) NK cells.7,8 ML NK cells have increased expression of activating receptors (eg, natural cytotoxicity receptors) and enhanced activity against AML cells, regardless of killer immunoglobulin-like receptor (KIR)/HLA inhibitory interactions.
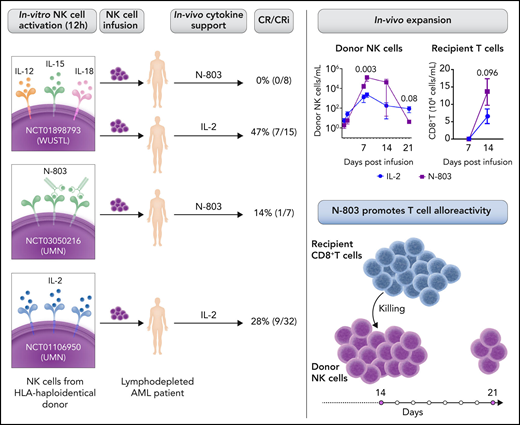
In independent clinical trial cohorts of patients with AML who were treated with adoptive haploidentical NK (haplo-NK) cell immunotherapy, Berrien-Elliott et al observed worse outcomes in patients receiving the support of N-803 compared with IL-2. These clinical results were unexpected and further investigated. N-803 promoted a more robust early expansion of infused NK cells than IL-2, but the in vivo persistence was limited. Because the NK cell phenotype was similar in N-803 and IL-2 cohorts, other factors could have contributed to the low clinical response. Indeed, the frequency and proliferation of host CD8+ T cells was increased in patients with N-803 support. In vitro mixed lymphocyte reaction experiments demonstrated that IL-15/N-803 induced higher T-cell proliferation and activation than IL-2 and resulted in enhanced killing of allogeneic NK target cells. These data indicate that N-803 administration induces recipient CD8+ T-cell alloresponses that may limit the persistence of the infused NK cells (see figure).
Summary of clinical trials from WUSTL (Washington University) and UMN (University of Minnesota) studying cohorts of patients with AML treated with adoptive haploidentical NK cell therapy with N-803 or IL-2 support and their outcome. In vivo expansion of donor NK and recipient CD8+ T-cell frequency at the indicated days after NK cell infusion (WUSTL cohort) (see Figure 1C and 1I in the article by Berrien-Elliott et al).1 The low persistence of infused NK cells is related to recipient CD8+ T cell-mediated alloresponses induced by IL-15/N-803. Professional illustration by Somersault18:24.
Summary of clinical trials from WUSTL (Washington University) and UMN (University of Minnesota) studying cohorts of patients with AML treated with adoptive haploidentical NK cell therapy with N-803 or IL-2 support and their outcome. In vivo expansion of donor NK and recipient CD8+ T-cell frequency at the indicated days after NK cell infusion (WUSTL cohort) (see Figure 1C and 1I in the article by Berrien-Elliott et al).1 The low persistence of infused NK cells is related to recipient CD8+ T cell-mediated alloresponses induced by IL-15/N-803. Professional illustration by Somersault18:24.
This report shares the results of clinical trials from two medical centers and provides an explanation of why IL-15/N-803 negated the expectations of positively impacting adoptive haplo-NK cell therapy. The sustained serum levels of IL-15 generated by subcutaneous N-803 administration provide optimal support not only for the donor-derived activated NK cells but also for the host alloreactive CD8+ T cells. Therefore, the therapeutic NK cells infused were rejected, leading to a worse clinical outcome compared with IL-2. Thus, IL-15/N-803 is a double-edged sword. The high degree of HLA incompatibility in the haploidentical setting can be an advantage for NK cell antitumor activity but is extremely treacherous for promoting T-cell alloreactivity. Because haploidentical donors can be readily available for providing cellular therapy to patients with hematological malignancies, this is a disappointment. Haploidentical HSCT (haplo-HSCT) provides life-saving therapy for high-risk patients with leukemia who do not have HLA-matched donors. However, strategies have been developed to avoid patient and donor reciprocal T-cell alloreactivity, which is mainly responsible for graft rejection and graft-versus-host disease (GVHD).9 NK cells quickly reconstitute and can directly kill leukemia blasts without prior sensitization, thereby exerting a graft-versus-leukemia effect, which is dissociated from GVHD. NK cells can be alloreactive, according to the expression of inhibitory KIR(s) specific for HLA allotypes present in the donor and absent in the patient cells.9 In a haplo-HSCT setting, the adoptive immunotherapy using ML-NK cells, derived from the same donor and supported by N-803, may be well tolerated and characterized by efficient and persistent antileukemia activity (NCT02782546). In a nontransplantation setting, adoptive transfer of haplo-NK cells requires careful attention to avoid high doses of systemic IL-15/N-803 and/or to possibly use a more intense lymphodepleting chemotherapy.
Approaches of adoptive NK cell immunotherapy need to be tailored for optimal NK cell activity and reduced host T-cell–mediated allorejection to preserve NK cell expansion and persistence. Important factors to take into consideration include the type of allogeneic donor, the HLA-matched or HLA-mismatched donor/recipient pair, the cytokine(s) used in vitro to preactivate NK cells, and the cytokine protocols in vivo to sustain NK cells. In addition, the development of safer and more selective preparatory conditioning regimens should be pursued to improve and expand the clinical use of adoptive NK cell therapy.
Conflict-of-interest disclosure: D.P. and R.M. declare no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal