Issue Archive
Table of Contents
BLOOD COMMENTARIES
SPECIAL REPORT
Sickle cell trait does not cause “sickle cell crisis” leading to exertion-related death: a systematic review
Clinical Trials & Observations
This Special Report by Weeks and colleagues discusses how the American Society of Hematology convened an expert panel of hematologists and forensic pathologists to investigate the relationship between sickle cell trait (SCT), sickle cell pain crises, and exertion-related mortality. The panel conducted a systematic review of the literature and found no evidence supporting an association between SCT and sudden unexplained death, except in cases involving exertion-related rhabdomyolysis. Therefore, SCT should not be considered a cause of sudden unexplained death or exertion-related death unless rhabdomyolysis is present.
BLOOD SPOTLIGHT
Impact of new medications on the treatment of immune TTP
In recent years, 2 new therapies have been approved for thrombotic thrombocytopenic purpura (TTP): caplacizumab for immune TTP and recombinant ADAMTS13 for congenital TTP. In this Blood Spotlight, Scully et al review the potential impact of these treatments in improving acute TTP outcomes, reducing the need for plasma therapy and shortening the time to clinical response.
HOW I TREAT
How I diagnose and treat acute infection–associated purpura fulminans
Purpura fulminans (PF) is a rare but severe complication of sepsis, characterized by disseminated intravascular coagulation with a distinct thrombotic phenotype that leads to significant morbidity and mortality due to uncontrolled thrombosis. Bendapudi and Losman explore the pathophysiology and clinical features of PF and propose a treatment algorithm. Managing PF is challenging due to limited clinical data; however, recognizing its underlying cause—a failure of the protein C pathway—can guide the development of rational treatment strategies.
CLINICAL TRIALS AND OBSERVATIONS
Reducing clinical trial eligibility barriers for patients with MDS: an icMDS position statement
Clinical Trials & Observations
Overly stringent eligibility criteria can impede patient accrual in clinical trials, particularly in diseases where patients are older and more likely to have comorbidities. Borate et al, on behalf of the International Consortium for Myelodysplastic Syndromes (icMDS), examined trial eligibility for patients with MDS and found that exclusion criteria in most trials were often unrelated to drug safety concerns. After a Delphi process among MDS experts, the authors present practical guidelines for designing more inclusive and drug-specific eligibility criteria, which should enhance patient access to MDS trials.
Itacitinib for prevention of graft-versus-host disease and cytokine release syndrome in haploidentical transplantation
Clinical Trials & Observations
Haploidentical hematopoietic cell transplantation (haplo-HCT) with posttransplant cyclophosphamide is increasingly utilized, but complications such as graft-versus-host disease (GVHD) and cytokine release syndrome (CRS) remain significant concerns. Abboud and colleagues report the addition of the JAK1 inhibitor itacitinib to a standard GVHD prophylaxis regimen after haplo-HCT in 42 patients. The authors found that it effectively prevented CRS, with no cases of grade 2 to 5 CRS, and significantly reduced GVHD incidence, especially grade 3 to 4 GVHD. These promising results, if confirmed in a randomized trial, could lead to a change in clinical practice.
IMMUNOBIOLOGY AND IMMUNOTHERAPY
Two-layered immune escape in AML is overcome by Fcγ receptor activation and inhibition of PGE2 signaling in NK cells
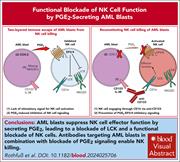
Natural killer (NK) cells reconstitute early after allogeneic hematopoietic stem cell transplantation and play a crucial role in the graft-versus-leukemia effect against acute myeloid leukemia (AML). Rothfuß et al investigated mechanisms of immune escape and found that AML blasts secrete prostaglandin E2 (PGE2), which functionally impairs NK cells by inhibiting lymphocyte-specific protein tyrosine kinase–dependent activation. Notably, blockade of PGE2 signaling restores NK cell cytotoxicity, raising the possibility of integrating pharmacological strategies targeting this axis with NK cell–based therapies to enhance anti-AML immune responses.
LYMPHOID NEOPLASIA
Targeting WDR5/ATAD2 signaling by the CK2/IKAROS axis demonstrates therapeutic efficacy in T-ALL
While epigenome-targeting therapies have shown significant therapeutic benefits in various hematological malignancies, no such treatments are currently available for T-cell acute lymphoblastic leukemia (T-ALL). Han et al identified a novel epigenomic targeting strategy in T-ALL by inhibiting the essential histone modifier WD repeat-containing protein 5 (WDR5), a chromatin-associated WD40 repeat domain protein. Additionally, the authors demonstrate that combining a direct WDR5 inhibitor with casein kinase II (CK2) inhibition, which indirectly reduces WDR5 expression, leads to synergistic antileukemic effects. These findings provide a rationale for clinical evaluation of epigenome-targeting approaches in T-ALL therapy.
MYELOID NEOPLASIA
α-Ketoglutarate dehydrogenase is a therapeutic vulnerability in acute myeloid leukemia
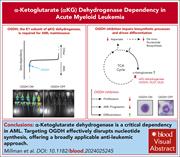
Metabolic dependencies in acute myeloid leukemia (AML) have been explored as therapeutic targets for metabolism-focused treatment strategies. Millman et al identify gene-encoding components of the α-ketoglutarate dehydrogenase complex, including 2-oxoglutarate dehydrogenase (OGDH), as specific genotype-independent dependencies in AML. The authors demonstrate that OGDH depletion impairs leukemic progression and extends survival in models of MLL-fusion and p53-mutant AML. While translating metabolic findings from preclinical models remains challenging, this study underscores the therapeutic potential of targeting OGDH in AML treatment.
BLOOD WORK
-
Cover Image
Cover Image
![issue cover]()
Characteristic fibrin-rich microvascular thrombi within small vessels of the dermis, consistent with disseminated intravascular coagulopathy, in a patient with purpura fulminans. See the article by Bendapudi and Losman on page 1358.
- PDF Icon Front MatterFront Matter
- PDF Icon Table of ContentsTable of Contents
- PDF Icon Editorial BoardEditorial Board
Advertisement intended for health care professionals
Email alerts
Advertisement intended for health care professionals









Jumping barriers in clinical trials: release the brake
Clinical Trials & Observations