Issue Archive
Table of Contents
BLOOD COMMENTARIES
HOW I TREAT
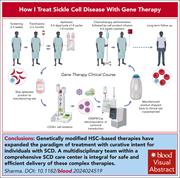
How I treat sickle cell disease with gene therapy
New treatment options are becoming available for people with severe sickle cell disease with the US Food and Drug Administration’s approval of 2 autologous gene therapy products. Akshay Sharma illustrates key considerations for physicians and patients in 3 informative cases. This article provides timely practical advice on candidate selection, product selection, cell collection, acute care, and long-term follow up.
CLINICAL TRIALS AND OBSERVATIONS
Sustained benefit of zanubrutinib vs ibrutinib in patients with R/R CLL/SLL: final comparative analysis of ALPINE
Clinical Trials & Observations
HEMATOPOIESIS AND STEM CELLS
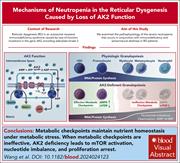
Failure of metabolic checkpoint control during late-stage granulopoiesis drives neutropenia in reticular dysgenesis
Reticular dysgenesis is 1 of the most devastating severe immunodeficiency syndromes, characterized by severe neutropenia, T- and natural killer–cell lymphopenia, and a lack of both innate and adaptive immune function. Wang et al used single cell transcriptomics to identify expanded populations of progenitor cells but relatively selective deficiency of bone marrow granulocytic precursors. The authors pinpointed deregulation of energy metabolism in maturing myeloid cells due to deficiency of adenylate kinase 2 as the cause of the severe neutropenia and increase the knowledge of the regulation of neutrophil energy production.
IMMUNOBIOLOGY AND IMMUNOTHERAPY
Deletions in the MAL gene result in loss of Mal protein, defining the rare inherited AnWj-negative blood group phenotype
Human blood groups are inherited polymorphisms of erythrocyte surface proteins that can, via transfusion or pregnancy, stimulate the production of alloantibodies. Tilley and colleagues reveal their discovery of the gene underlying the inherited AnWj-negative red cell phenotype, identifying a 6646 base pair deletion at the MAL locus in 8 AnWj-negative individuals but not in AnWj-positive family members. The authors also identified that not all AnWj-negative phenotypes were inherited and that loss of expression of Mal can occur through yet unproven mechanisms. These data underpin recognition of AnWj as a blood group system and will prompt further investigation of the function of the Mal protein.
LYMPHOID NEOPLASIA
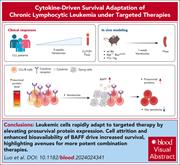
Venetoclax dose escalation rapidly activates a BAFF/BCL-2 survival axis in chronic lymphocytic leukemia
While highly potent at killing chronic lymphocytic leukemia (CLL) cells, treatment with the B-cell lymphoma-2 (BCL-2) inhibitor venetoclax does not clear all leukemic cells. Luo et al studied patients initiated on obinutuzumab-venetoclax and genetically engineered mice to describe a mechanism by which a small minority of CLL cells survived during the venetoclax ramp-up phase. B-cell depletion induced by obinutuzumab promoted the production of cytokine B-cell activating factor (BAFF), which in turn promoted CLL survival despite ongoing venetoclax therapy by increasing both mantle cell lymphoma-1 and BCL-2 expression. These data provide a strong clue to future combination therapies designed to block this BAFF-mediated adaptation.
RED CELLS, IRON, AND ERYTHROPOIESIS
TR4 and BCL11A repress γ-globin transcription via independent mechanisms
Upregulating γ-globin gene expression to raise fetal hemoglobin is highly desirable in severe sickle cell disease and transfusion-dependent β-thalassemia. In searching for novel ways to achieve this, Wang et al identified that the orphan nuclear receptor TR4 competes with the known γ-globin repressor BCL11 transcription factor A (BCL11A) at overlapping DNA sequences in the promoter region. These data clear the way for the pursuit of new strategies to modulate fetal hemoglobin levels in patients.
TRANSPLANTATION
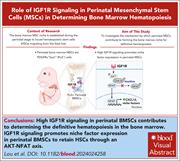
IGF1R signaling in perinatal mesenchymal stem cells determines definitive hematopoiesis in bone marrow
Having been maintained in the fetal liver during gestation, hematopoietic stem cells (HSCs) migrate to the perinatal bone marrow to establish definitive hematopoiesis by a mechanism that has proven elusive. Lou and coauthors implicate insulin-like growth factor 1 receptor (IGF1R) signaling in this initial HSC retention by the nascent bone marrow niche, while showing that it is redundant in adult bone marrow. Mechanistically, the authors show that this signaling upregulates the chemokine CXCL12 by bone marrow stromal cells.
LETTER TO BLOOD
Refined ELN 2024 risk stratification improves survival prognostication following venetoclax-based therapy in AML
BLOOD WORK
-
Cover Image
Cover Image
![issue cover]()
Transmission electron microscopic image of representative mitochondria from adenylate kinase 2–deficient hematopoietic stem and progenitor cells differentiated in vitro into colony forming unit–granulocytes. The elongated shape with tightly folded cristae is typical of cells under increased oxidative phosphorylation demand. See the article by Wang et al on page 2718.
- PDF Icon Front MatterFront Matter
- PDF Icon Table of ContentsTable of Contents
- PDF Icon Editorial BoardEditorial Board
Advertisement intended for health care professionals
Email alerts
Advertisement intended for health care professionals