In this issue of Blood, Wang et al revisit the important topic of γ-globin gene repression in adult erythropoiesis, and the role played by TR4, an orphan steroid hormone receptor.1
The identity of transcriptional repressors that bind the distal CCAAT box motif in the duplicated γ-globin gene promoters has been of interest for many decades, because naturally occurring mutations in this region result in hereditary persistence of fetal hemoglobin (HPFH) in adult red blood cells via disruption of binding of these repressors. HPFH ameliorates the severity of sickle cell disease (SCD) and transfusion-dependent β-thalassemia, so the mechanisms that underpin physiological repression of HBG expression have major therapeutic implications. Wang et al reinvestigate the role of the imperfect direct repeat element (DR1; AGGTCAATAGCCT), which overlaps the CCAAT box. The DR1 motif is disrupted by the −117-, −114-, and 13-base pair deletion HPFH mutations. The authors reexamined the role played by the orphan nuclear hormone receptor TR4 (NR2C2) in repression of HBG via binding DR1 (see figure). TR4 was initially purified from erythroid cell lines using DNA-binding assays for DR1.2 Other authors found another orphan hormone receptor, COUP-TFII,3 via purification of a similar DNA-binding activity known as nuclear factor E3.
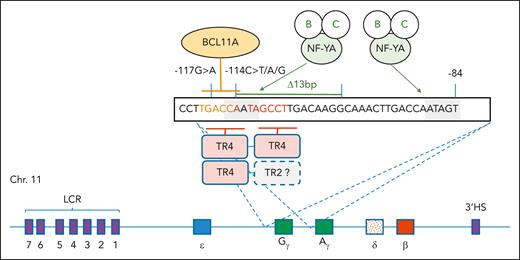
The model shows the sequence motifs and DNA-binding proteins that mediate transcriptional regulation of HBG1 and HBG2. Organization of the human β-globin locus on chromosome 11 is at the bottom, with the 5 functional genes color coded by site of expression in the yolk sac (ε), fetal liver (Gγ and Aγ), and adult bone marrow (δ and β) (not to scale). The presence of the 3′ HS site and the core HS sites within the LCR are shown as purple boxes. The 2 CCAAT boxes are shaded, and binding to them by the NF-Y complex (A, B, and C components) is indicated. BCL11A binds to the TGACC motif highlighted in orange, and TR4 (as homo- or heterodimers with other steroid hormone receptors) binds to the DR1 sequence, which is underlined and highlighted with red/orange text. The positions of the −117 and −114 and Δ13 hereditary persistence of fetal hemoglobin mutations are indicated. NF-YA, nuclear factor Y, component A.
The model shows the sequence motifs and DNA-binding proteins that mediate transcriptional regulation of HBG1 and HBG2. Organization of the human β-globin locus on chromosome 11 is at the bottom, with the 5 functional genes color coded by site of expression in the yolk sac (ε), fetal liver (Gγ and Aγ), and adult bone marrow (δ and β) (not to scale). The presence of the 3′ HS site and the core HS sites within the LCR are shown as purple boxes. The 2 CCAAT boxes are shaded, and binding to them by the NF-Y complex (A, B, and C components) is indicated. BCL11A binds to the TGACC motif highlighted in orange, and TR4 (as homo- or heterodimers with other steroid hormone receptors) binds to the DR1 sequence, which is underlined and highlighted with red/orange text. The positions of the −117 and −114 and Δ13 hereditary persistence of fetal hemoglobin mutations are indicated. NF-YA, nuclear factor Y, component A.
BCL11A binds the DNA sequence, TGACC, which also overlaps the CCAAT box and half of the DR1 element (see figure), so there is potential for complex interactions between BCL11A, TR4, and possibly other factors that bind in the vicinity of the CCAAT box, including the nuclear factor Y complex (see figure).4 HPFH mutations also disrupt binding of BCL11A (see figure).5 When crossed to a mouse model of human SCD, a genetic knockout of Bcl11A results in marked upregulation of human HBG and cure.6 However, BCL11A is also expressed in nonerythroid cells, including HSCs and brain cells. A complete knockout of Bcl11A in HSCs damages their health.7 Remarkably, a precision CRISPR editing of the +58 intronic enhancer is sufficient to markedly increase expression of HBG and production of fetal hemoglobin (HbF) in primary erythroid cells to therapeutic levels without significantly effecting other blood lineages.8 This discovery led to the commercial development of a CRISPR/Cas9-based autologous hematopoietic stem cell therapy known as exagamglogene autotemcel (Casgevy, Vertex Pharmaceuticals), which was recently granted approval from the US Food and Drug Administration for treatment of SCD and transfusion-dependent β-thalassemia based on 2 very successful clinical trials.9,10
In their study, Wang et al show how TR4 competes with BCL11A for binding to the overlapping DR1 and BCL11A motifs using electromobility shift assays. They show CRISPR/Cas9 gene editing of TR4 results in marked upregulation of HBG expression, F-cell numbers to >40%, and HbF production to >20% of total hemoglobin by high-performance liquid chromatography in HUDEP-2 cells.8 Most interestingly, upregulation of HBG expression in BCL11A CRISPR-edited clones was rescued by forced overexpression of TR4. This result suggests TR4 may be a mediator of the reactivation of HBG gene expression in BCL11A knockout or knockdown cells, or that BCL11A and TR4 may work together somehow to mediate physiological repression of HBG1 and HBG2 in adult erythroid cells. However, this overexpression experiment does not prove TR4 plays this repression role in primary cells. Wang et al’s experiments in primary CD34-derived erythroid cells were less convincing in showing a role for TR4, but this may have been due to less efficacy of the gene editing.
Wang et al also performed chromatin immunoprecipitation sequencing (ChIP-seq) assays with epitope-tagged versions of TR4 and BCL11A in HUDEP-2 cells. In these experiments, the endogenous genes were tagged, so the expression was physiological. They found that BCL11A binds weakly to the HBG promoters and strongly to all hypersensitivity sites (HSs) within the locus control region (LCR; see Wang et al, Figure 5), and TR4 binds weakly to the HBG promoters and quite strongly to HS2 of the LCR. Quantification of ChIP-seq data of this nature is difficult, but one possible interpretation is that TR4 helps recruit or retain BCL11A at the HBG promoters. It would be of interest to test this by undertaking ChIP for BCL11A in cells lacking TR4.
Wang and colleagues remind us of the complexity of γ-globin gene repression. They show that TR4 plays a role, but there remain unanswered questions about the mechanism by which it acts. Do BCL11A and TR4 antagonize each other as suggested by the competitive electrophoretic mobility shift assays, work together, or work independently to repress γ-globin gene expression as suggested by the loss of function and rescue assays? Does TR4 regulate other genes in erythroid cells that play indirect roles in HBG repression? What role does displacement of the CCAAT-binding protein complex nuclear factor Y play in repression of HBG by TR4 and BCL11A? Does TR4 heterodimerize with other steroid hormone receptors at the DR1 element in vivo? What role does TR4 binding to the LCR play? Answers to these questions can be obtained with further experimentation.
Conflict-of-interest disclosure: A.C.P. declares no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal