In this issue of Blood, Luo et al1 enhance our understanding of the early adaptations of chronic lymphocytic leukemia (CLL) cells during the initial ramp-up period of venetoclax treatment, thereby providing valuable new insights into the therapeutic response.
CLL is the most common leukemia in adults worldwide and is characterized by the presence of long-lived CD19+CD5+ malignant B cells that exhibit a defect in apoptosis due to the elevated expression of antiapoptotic proteins of the BCL-2 family.2 Venetoclax, a potent and selective BCL-2 inhibitor, has emerged as a promising therapeutic strategy in CLL, demonstrating high treatment efficacy. However, despite high response rates, minimal residual disease and therapy resistance remain significant challenges.3 Mechanisms of venetoclax resistance, such as the upregulation of antiapoptotic genes, alterations in metabolic pathways and mitochondrial structures, genetic aberrations, epigenetic modifications of BCL-2 family genes, and alterations in signaling pathways, have been studied extensively.4,5 Nonetheless, the mechanisms underlying incomplete responses to venetoclax remain poorly understood.
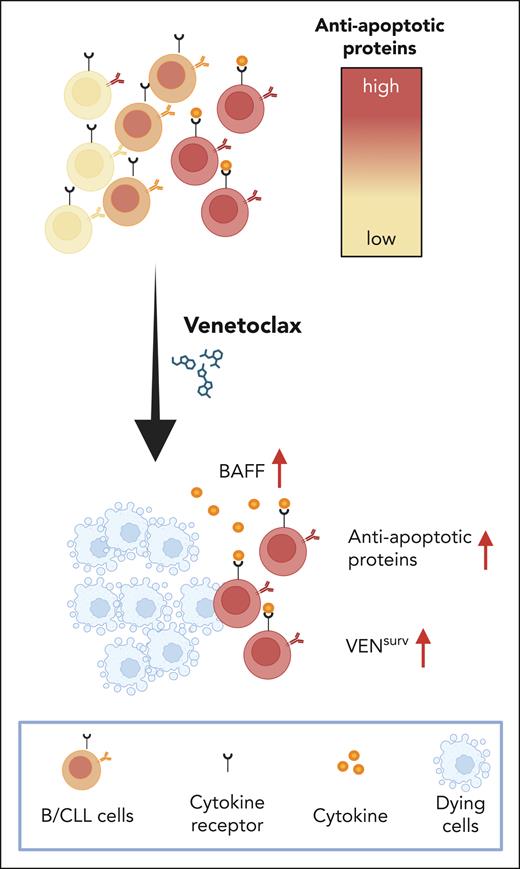
In their study, Luo et al address this knowledge gap. Using mass cytometry, they identified 10 distinct B/CLL-cell clusters, emphasizing the great heterogeneity within CLL cells. Interestingly, cells from all these CLL clusters responded uniformly to venetoclax treatment in a dose-independent manner. Further analysis revealed that short-term venetoclax exposure led to an increase in BCL-2 levels in the surviving cells, along with a slight upregulation of MCL-1 and BCL-XL proteins. Experiments performed in different mouse models demonstrated that ongoing apoptosis is required for this BCL-2 increase in surviving cells (VENsurv), recapitulating the responses observed in cells of patients with CLL. The factor that increased in serum after venetoclax treatment in wild-type mice, but not in mice with B lymphocytes unable to undergo apoptosis, was identified as B-cell–activating factor (BAFF). This factor promotes the maturation and survival of peripheral B cells by binding to the BAFF receptor (BAFF-R).6 To determine whether BAFF is responsible for the increase in BCL-2 levels in VENsurv cells, the authors transplanted apoptosis-resistant B cells together with B cells additionally lacking BAFF-R, into wild-type mice, followed by venetoclax treatment. Interestingly, an increase in BCL-2 was detected in apoptosis-resistant B cells, but not in BAFF-R–deficient B cells. A moderate increase in MCL-1 was also observed in both B-cell populations. These findings indicate that the increase in BCL-2 levels in VENsurv B cells is dependent on BAFF signaling, whereas the increase in MCL-1 occurs independently of BAFF. A similar mechanism was observed in human B cells from patients with metastatic breast cancer undergoing venetoclax treatment. The overall reduction in B-cell numbers was accompanied by elevated plasma levels of BAFF and a proliferation-inducing ligand (APRIL), along with increased BCL-2 expression in the remaining B cells. Transcriptome analysis of B cells after venetoclax treatment, using cellular indexing of transcriptomes and epitope sequencing of single cells, revealed enrichment in 17 pathways, including BAFF-related NF-κB signaling. Thijssen et al also reported NF-κB activation in CLL cells during the first year of venetoclax treatment,7 and Endo et al described BAFF-dependent NF-κB2 (p52) activation in CLL cells.8 Finally, Luo et al demonstrate that patients receiving venetoclax with dose escalation had higher levels of BAFF in the serum, and surviving CLL cells had increased levels of BCL-2 protein. In addition, in vitro experiments performed on treatment-naive CLL cells supported the link between venetoclax sensitivity and BAFF, and BCL-2. Furthermore, the authors show that patients treated with obinutuzumab, an anti-CD20 antibody that targets and eliminates CLL cells through a BCL-2–independent mechanism, exhibited elevated serum levels of BAFF and increased levels of BCL-2 in the surviving CLL cells. These findings imply that the observed effects are not exclusively related to BCL-2 inhibition. Rather, they indicate that the reduction in the leukemic cell burden leads to increased access to BAFF, which subsequently promotes survival of the residual cells and modulates their response to therapy (see figure).
Venetoclax treatment effectively reduces all CLL subpopulations; however, it simultaneously triggers a rapid upregulation of antiapoptotic proteins, including BCL-2, BCL-XL, and MCL-1, in the surviving cells, thereby diminishing their sensitivity to the drug. This upregulation of antiapoptotic proteins is dependent on a significant depletion of CLL cells and requires access to the B-cell cytokine BAFF. These findings suggest that the sensitivity of CLL cells to venetoclax is mediated by a cytokine-dependent mechanism, highlighting the importance of targeting cytokine signals to enhance therapeutic efficacy. Created with BioRender.com.
Venetoclax treatment effectively reduces all CLL subpopulations; however, it simultaneously triggers a rapid upregulation of antiapoptotic proteins, including BCL-2, BCL-XL, and MCL-1, in the surviving cells, thereby diminishing their sensitivity to the drug. This upregulation of antiapoptotic proteins is dependent on a significant depletion of CLL cells and requires access to the B-cell cytokine BAFF. These findings suggest that the sensitivity of CLL cells to venetoclax is mediated by a cytokine-dependent mechanism, highlighting the importance of targeting cytokine signals to enhance therapeutic efficacy. Created with BioRender.com.
Luo and colleagues provide compelling evidence that CLL cells adapt rapidly to venetoclax treatment by upregulation of antiapoptotic genes through cytokine-mediated signaling, specifically involving BAFF. Several studies have highlighted the crucial role of BAFF in both CLL pathogenesis and venetoclax resistance,6,9,10 underscoring the need for further clinical study of this signaling axis. These findings emphasize the need to develop more effective combination therapies that can overcome resistance and improve patient outcomes.
Conflict-of-interest disclosure: The authors declare no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal